New publication in eLife of the Jordan Lab in collaboration with the IBMB Imaging Platform showing that…
The crystal structure of a 250-kDa heterotetrameric particle explains inhibition of sheddase meprin β by endogenous fetuin-B.
• Meprine-β is a metallopeptidase enzyme essential for cell signalling processes since it cuts and releases proteins in the cell membrane, thus solubilizing them to make them available to the body for various functions.
• Its malfunction could have implications in diseases such as Alzheimer’s, fibrosis, nephritis or cancer, in which proteins anchored in the cell membrane that are a substrate for β-meprin are involved.
A study by the Molecular Biology Institute of Barcelona (IBMB) of the CSIC and the Johannes-Gutenberg University of Mainz (Germany) manages to reveal an essential cellular mechanism in cell signalling, whose dysfunction could contribute to the development of neurodegenerative diseases such as Alzheimer’s, as well as fibrosis, nephritis or cancer. The research results are published in the journal Proceedings of the National Academy of Sciences USA.
The scientists of the IBMB-CSIC Ulrich Eckhard, first author of the article, and F. Xavier Gomis-Rüth, corresponding author, explain that they have managed to visualize the structure of an enzyme of the cell membrane, meprin-β, inhibited by another protein, fetuin B, through the technique of crystallization and X-ray diffraction. This inhibition, scientists say, prevents meprin-β from performing its normal function, which is to cut and release proteins (previously synthesized by the cell and transported to the surface of it) and make them available to the body for various functions.
Gomis-Rüth, Research Professor and head of the Proteolysis Laboratory at the IBMB-CSIC, explains that “meprin-β cuts (protein) substrates anchored in the membrane and thereby generates the soluble forms of these proteins so that they can perform endocrine and paracrine functions”.
Since β-meprin is located in cells of the central nervous system and the kidney, its dysregulation can lead to diseases related to these organs, such as nephritis, fibrosis, cancer, or neurodegenerative diseases such as Alzheimer’s.
The action of β-meprin is necessary to cut and activate forms of proteins that are anchored in the membrane. Gomis-Rüth points out that in the case of Alzheimer’s disease, “the connection of β-meprin with the disease is not known in detail, but it must play a role since in this case there is also a substrate protein of meprin-β, the amyloid precursor protein (APP), which is anchored in the cell membrane and which is the precursor of amyloidogenic peptides ”. The latter is the main component of the characteristic β-amyloid plaques that form in the brain tissue of patients with Alzheimer’s disease.
X-ray crystallography techniques have been used for the work. It is a complex technique to crystallize biological molecules so that they are immobilized. In this way, a three-dimensional still image of the molecules is obtained. To visualize this image and decipher its structure, X-ray diffraction is used, such as that generated in synchrotrons such as ALBA, in Cerdanyola del Vallés (Barcelona). In the case of the meprin-β structure inhibited by fetuin B, the researchers describe the latter as an “elephant with its trunk raised.” It is a simile, explains Gomis-Rüth, because we have seen that, of the areas of fetuin B that interact with β-meprin, one looks like the trunk and two more look like the front and rear legs, respectively ”.
With the mechanism derived from this structure, which has been complemented with numerous biochemical tests, the door is opened to new investigations to discern the detailed roles of β-meprin and fetuin B in the biological processes in which they are involved.
*Press release made by the communication staff of the CSIC Delegation in Catalonia.
Reference:
The crystal structure of a 250-kDa heterotetrameric particle explains inhibition of sheddase meprin β by endogenous fetuin-B
Ulrich Eckhard, Hagen Körschgen, Nele von Wiegen, Walter Stöcker, and F. Xavier Gomis-Rüth.
PNAS April 6, 2021 118 (14) e2023839118;
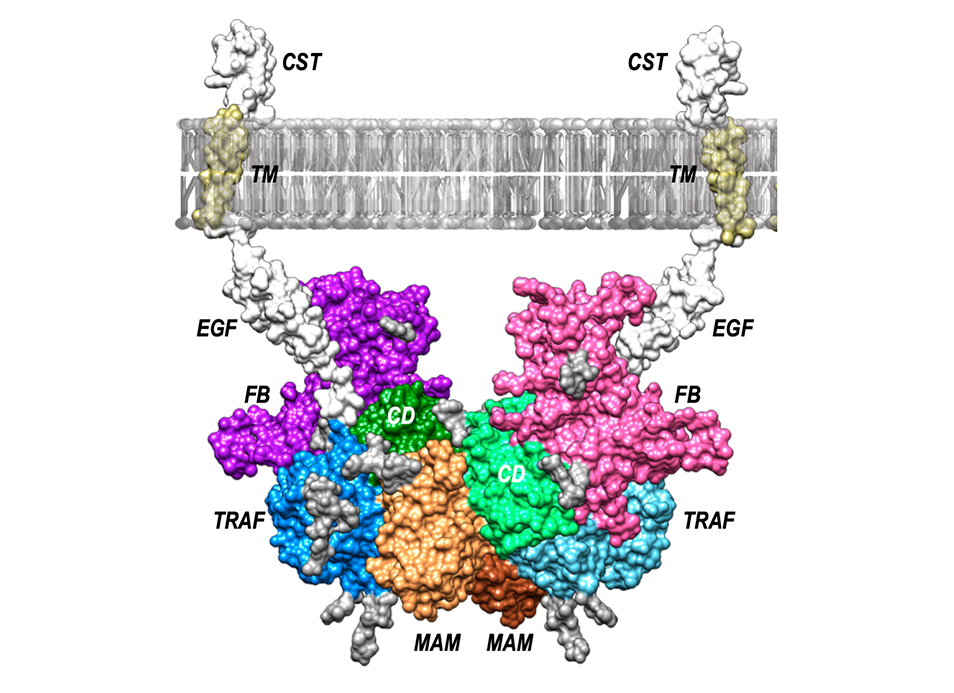
Legend for picture: Model of the arrangement of the heterotrimeric complex between the metallopeptidase meprin β dimer (domains in brown [MAM], light blue [TRAF] and light green [CD], as well as orange [MAM], blue [TRAF] and green [CD]) with two fetuin B (FB) moieties (in purple in pink). Anchorage to the cytoplasmic membrane is provided by the C-terminal meprin β domains EGF, TM and CST.