Lab presentation
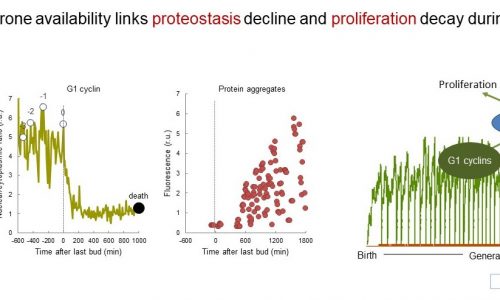
Protein misfolding and accumulation of proteotoxic aggregates has a direct impact in cell aging, but the underlying molecular mechanisms are poorly understood. Although it has been mainly associated to neurodegenerative diseases, recent evidence has linked proteostasis defects to cell precursor damage in proliferative tissues. As in many cell types of different origin, prion-like aggregates are asymmetrically distributed during cytokinesis in budding yeast, and we have found that their progressive accumulation through consecutive generations eventually inhibits cell cycle entry and leads the cell to an irreversible senescent state. Since prion-like and proteotoxic aggregates act as chaperone sinks, our data point to the idea that chaperome alterations would mediate the observed decline in cell proliferation during cell aging. With these hypotheses we aim at the identification of key molecules and mechanisms that restrain cell proliferation during cell aging and, hence, at a better understanding of aging at a systems level.
Closely related to the molecular interconnections between proteostasis machineries and cell fitness, the buildup of certain proteins into insoluble aggregates is a pathological hallmark of neurodegenerative diseases such as amyotrophic lateral sclerosis or Alzheimer disease. We are working to create antiprions, a new class of drugs to tackle these devastating pathologies. Just like the opposing twins Prometheus and Epimetheus, we propose that antiprions can be originated from prion proteins by introducing mutations that prevent the pathological conformational change of the normal prion protein in trans. To this end we are combining extremely powerful screens for such prion mutants and state-of-the-art analytical methodologies to test their antiprion properties in neurons.
Finally, we are currently interested in emergent properties of molecular networks driven by intrinsic disorder, focusing our work on their implications in the spatial organization of molecular processes within the cell.
Projects
Research Lines:
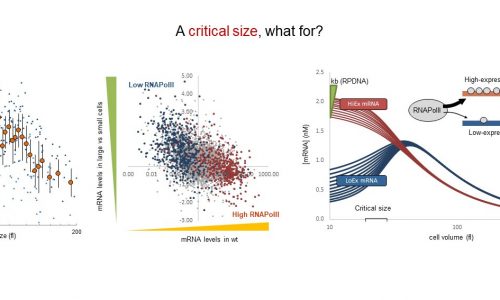
Prions and aggregons as START inhibitors: a path to cell aging
Protein misfolding and accumulation of proteotoxic aggregates has been mainly associated to neurodegenerative diseases, but there are also some evidences of damage to proliferative tissues. In the last two years we have accumulated enough experimental evidence to propose that prion-like aggregates act as inhibitors of cell proliferation in budding yeast, with direct consequences in cell aging and death. As in many cell types of different origin, prion-like aggregates are asymmetrically distributed during cytokinesis in budding yeast, and we have found that their progressive accumulation through consecutive generations eventually inhibits cell cycle entry and leads the cell to an irreversible senescent state. Since prion-like and proteotoxic aggregates act as chaperone sinks, our data suggest that chaperome alterations would mediate the observed decline in cell proliferation during cell aging. Here we propose (1) to perform a functional analysis of the chaperome under a variety of proteotoxic aggregation conditions; (2) to extend a genetic screen to identify proteotoxicity-protecting genes in aging cells; (3) to analyze the role of RNA-binding proteins and RNA-protein aggregates in cell aging, and (4) to complete an integrative analysis of candidate modulators of proteotoxic aggregation. With these objectives we aim at the identification of key molecules and mechanisms that restrain cell proliferation during cell aging and, hence, at a better understanding of aging at a systems level.
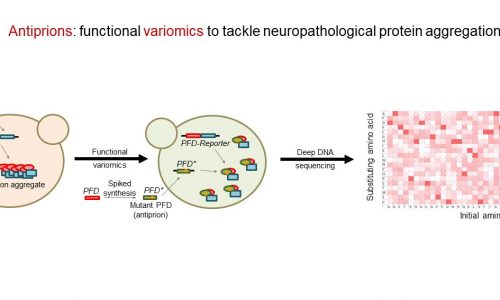
Antiprions: functional variomics to tackle neuropathological protein aggregation
Prions and prion-like aggregates have been directly implicated in more than 20 human diseases, among them neurodegenerative pathologies such as Alzheimer’s, Parkinson’s and Huntington’s diseases, and amyotrophic lateral sclerosis. Prion and prion-like proteins are self-propagating protein isoforms that accumulate as large structure-driven aggregates. It is generally accepted that their accumulation in the human brain is a direct cause of neuronal degeneration. However, appropriate therapeutic approaches and effective treatments are largely lacking, and efforts to decrease the rate of prion-like aggregation with peptides have produced very limited results. Here we hypothesize that, similarly to the opposing twins Prometheus and Epimetheus, antiprions can be created from prion domains as quasi-twin structures that (1) still bind with high efficiency to prion aggregates but (2) do not transmit the pathological fold to newly recruited monomers, thus arresting prion aggregate growth. Giving support to this hypothesis, prion misfolding and aggregation takes place in successive steps of conformational change. However, the details of these transitions are largely unknown, making impossible the post hoc design of mutants based on predicted structural properties. For this reason, our proposal is grounded in a non-biased approach, and plans to use neuropathological prion sequences (Aβ42, αSyn and TDP-43) as initials seeds to perform (1) extensive and highly-sensitive variomics screens to identify newly-generated peptides with antiprion properties under three orthogonal selection scenarios, and (2) comprehensive functional assays of the isolated candidates to validate their ability to counteract prion-like aggregation and deleterious effects in neuronal morphology and physiology. The discovery and functional analysis of antiprions will constitute the foundational basis of preclinical and clinical assays directed to test and refine their use in major neurodegenerative diseases.
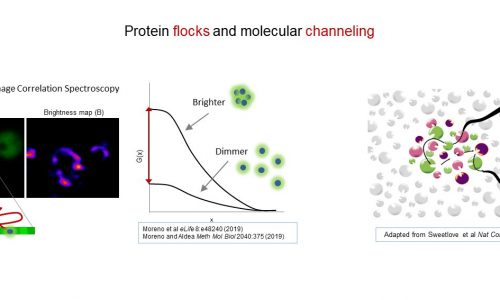
Protein cluster dynamics and network channeling
Whether sequential metabolic reactions and other complex step-wise intracellular processes are efficiently executed by proteins dispersed in a crowded environment is a matter of debate. While some researchers do not find necessary that enzymes need be in proximity to efficiently channel the reaction product from one enzyme to the next one as substrate, recent evidence points to the notion that, in many metabolic pathways, sequential enzymes would be organized in protein assemblies. However, the existence of stable interactions holding a large number of proteins all together in a keylock manner to perform a set of complex sequential reactions or processes is rather exceptional. Based on our previous work on single-molecule dynamics of two major yeast chaperones, here we test the hypothesis that functional channeling in protein networks could be driven by very transient and somewhat promiscuous protein-protein interactions. Our preliminary data suggest that these protein clusters would display extremely fast assembly and disassembly dynamics, well beyond those of membrane-less organelles. We will use single-molecule fluctuation analysis to study the spatiotemporal coincidence of 10000 protein pairs involved in different metabolic and signaling pathways, aiming at visualizing for the first time the protein clusters that are thought to drive molecular channeling. To confirm the existence of these dynamic protein assemblies, we will apply an orthogonal approach that we have started to develop to quantify local cluster fluctuations in vivo and in cell-free extracts. High-throughput proximity-labeling methods will allow us to analyze further the specificity and composition of the identified clusters. Finally, we will use the armamentarium of molecular and cellular biology tools offered by the budding yeast to study the relevance of cluster identity in the efficiency of paradigmatic pathways and networks.
Lab people

Martí Aldea
Principal investigator
After receiving my Ph.D. degree in Biological Sciences from the Autonomous University of Barcelona, I did postdoctoral work with Dr. Miguel Vicente at the Center of Biological Research (CSIC, Madrid) and with Dr. Sidney Kushner at the University of Georgia (USA), trying to understand the mechanisms that coordinate growth and cell division in Escherichia coli. I joined the University of Lleida to initiate my career as an independent scientist focusing my work on the control of the earliest steps of the cell cycle, willing to understand how cells control proliferation and growth in budding yeast, the first eukaryote where cyclins had been cloned in the early 90’s.
During this period, we discovered and deeply characterized a cytoplasmic-retention mechanism that controls the cell cycle in G1. Later work at the IBMB lead us to identify a molecular switch for cell cycle entry, and postulate for the first time a molecular theory for cell size control, a prominent biological problem since the start point of modern biology in the 50’s. As a result of the most recent work, my research group has uncovered an unexpected link between protein aggregation and cell cycle entry, pointing to proteostasis decline as a key factor causing cell proliferation decay during aging.
Past Students
Pedro Jesus Vidal
Alexis Pérez
Laia Ferrer
David Moreno
Selected publications
Pérez AP, Artés MH, Moreno DF, Clotet J, Aldea M. (2022). Mad3 modulates the G1 Cdk and acts as a timer in the Start network. Science Advances 8. doi: 10.1126/sciadv.abm4086
Yahya G, Pérez AP, Mendoza MB, Parisi E, Moreno DF, Artés MH, Gallego C, Aldea M. (2021). Stress granules display bistable dynamics modulated by Cdk. Journal of Cell Biology 220. doi: 10.1083/jcb.202005102
Moreno DF, Jenkins K, Morlot S, Charvin G, Csikasz-Nagy A, Aldea M. (2019). Proteostasis collapse, a hallmark of aging, hinders the chaperone-Start network and arrests cells in G1. eLife 8. doi: 10.7554/eLife.48240
Martínez-Láinez JM, Moreno DF, Parisi E, Clotet J, Aldea M. (2018). Centromeric signaling proteins boost G1 cyclin degradation and modulate cell size in budding yeast. PLoS Biology 16. doi: 10.1371/journal.pbio.2005388
Parisi E, Yahya G, Flores A, Aldea M. (2018). Cdc48/p97 segregase is modulated by Cdk to determine cyclin fate during G1 progression. The EMBO Journal 37. doi: 10.15252/embj.201798724
Aldea M, Jenkins K, Csikász-Nagy (2017). Growth Rate as a Direct Regulator of the Start Network to Set Cell Size. Frontiers in Cell and Developmental Biology 5. doi: 10.3389/fcell.2017.00057
Saarikanga J, Caudron F, Prasad R, Moreno DF, Bolognesi A, Aldea M, Barral Y. (2017). Compartmentalization of ER-bound chaperone confines protein deposit formation to the aging yeast cell. Current Biology 27. doi: 10.1016/j.cub.2017.01.069
Georgieva MV, Yahya G, Codó L, Ortiz R, Teixidó L, …, Gelpí JL, Gallego C, Orozco M, Aldea. (2015). Inntags: small self-structured epitopes for innocuous protein tagging. Nature Methods 12: 955–958. doi: 10.1038/nmeth.3556
Yahya G, Parisi E, Flores A, Gallego C, Aldea M. (2014). A Whi7-anchored loop controls de G1 Cdk-cyclin complex at Start. Molecular Cell 53: 115-126. doi: 10.1016/j.molcel.2013.11.015
Menoyo S, Ricco N, Bru S, Hernández-Ortega S, Escoté X, Aldea M, Clotet J. (2013). Phosphate-activated CDK stabilizes G1 cyclin to trigger cell cycle entry. Molecular and Cellular Biology 33: 1273-84. doi: 10.1128/MCB.01556-12
Ferrezuelo F, Colomina N, Palmisano A, Garí E, Gallego C, Csikasz-Nagy A, Aldea M. (2012). The critical size is set at a single-cell level by growth rate to attain homeostasis and adaptation. Nature Communications 3: 1012 p1-11. doi: 10.1038/ncomms2015
Ferrezuelo F, Colomina N, Futcher B, Aldea M. (2010) The transcriptional network activated by Cln3 cyclin at the G1-to-S transition of the yeast cell cycle. Genome Biology 11: R67 p1-18. doi: 10.1186/gb-2010-11-6-r67
Vergés E, Colomina N, Garí E, Gallego C, Aldea M. (2007). Cyclin Cln3 is retained at the ER and released by the J-chaperone Ydj1 in late G1 to trigger cell cycle entry. Molecular Cell 26: 649-662. doi: 10.1016/j.molcel.2007.04.023
Clotet J, Escoté X, Adrover MA, Garí E, Aldea M, De Nadal E, Posas F. (2006). Phosphorylation of Hsl1 by Hog1 leads to a G2 arrest essential for cell survival at high osmolarity. The EMBO Journal 25: 2338-2346. doi: 10.1038/sj.emboj.7601095
Wang H, Garí E, Vergés E, Gallego C, Aldea M. (2004). Recruitment of Cdc28 by Whi3 restricts nuclear accumulation of the G1 cyclin-Cdk complex to late G1. The EMBO Journal 23: 180-190. doi: 10.1038/sj.emboj.7600022
Garí E, Volpe T, Wang H, Gallego C, Futcher B, Aldea M. (2001). Whi3 binds the mRNA of the G1 cyclin CLN3 to modulate cell fate in budding yeast. Genes & Development 15: 2803-2808. doi: 10.1101/gad.203501
Colomina N, Garí E, Gallego C, Herrero E, Aldea M. (1999). G1 cyclins block the Ime1 pathway to make mitosis and meiosis incompatible in budding yeast. The EMBO Journal 18: 320-329. doi: 10.1093/emboj/18.2.320
Gallego C, Garí E, Colomina N, Herrero E, Aldea M. (1997). The Cln3 cyclin is downregulated by translational repression and degradation during the G1 arrest caused by nitrogen deprivation in budding yeast. The EMBO Journal 16: 7196-7206. doi: 10.1093/emboj/16.23.7196
Dujon B, Albermann K, Aldea M, Alexandraki D, Ansorge W, et al. (1997). The nucleotide sequence of Saccharomyces cerevisiae chromosome XV. Nature 387(Suppl.): 98-102. doi: 10.1038/387s098
All publications
Pérez AP, Artés MH, Moreno DF, Clotet J, Aldea M. (2022). Mad3 modulates the G1 Cdk and acts as a timer in the Start network. Science Advances 8. doi: 10.1126/sciadv.abm4086
Yahya G, Pérez AP, Mendoza MB, Parisi E, Moreno DF, Artés MH, Gallego C, Aldea M. (2021). Stress granules display bistable dynamics modulated by Cdk. Journal of Cell Biology 220. doi: 10.1083/jcb.202005102
Moreno DF, Jenkins K, Morlot S, Charvin G, Csikasz-Nagy A, Aldea M. (2019). Proteostasis collapse, a hallmark of aging, hinders the chaperone-Start network and arrests cells in G1. eLife 8. doi: 10.7554/eLife.48240
Moreno DF, Parisi E, Yahya G, Vaggi F, Csikász-Nagy A, Aldea M (2019) Competition in the chaperone-client network subordinates cell-cycle entry to growth and stress. Life Science Alliance 2:e201800277. doi: 10.26508/lsa.201800277
Moreno DF, Aldea M (2019) Coincidence analysis of molecular dynamics by raster-image correlation spectroscopy. Methods in Molecular Biology 2040: 375-84. doi: 10.1007/978-1-4939-9686-5_17
Martínez-Láinez JM, Moreno DF, Parisi E, Clotet J, Aldea M. (2018). Centromeric signaling proteins boost G1 cyclin degradation and modulate cell size in budding yeast. PLoS Biology 16. doi: 10.1371/journal.pbio.2005388
Parisi E, Yahya G, Flores A, Aldea M. (2018). Cdc48/p97 segregase is modulated by Cdk to determine cyclin fate during G1 progression. The EMBO Journal 37. doi: 10.15252/embj.201798724
Saarikanga J, Caudron F, Prasad R, Moreno DF, Bolognesi A, Aldea M, Barral Y. (2017). Compartmentalization of ER-bound chaperone confines protein deposit formation to the aging yeast cell. Current Biology 27. doi: 10.1016/j.cub.2017.01.069
Aldea M, Jenkins K, Csikász-Nagy (2017). Growth Rate as a Direct Regulator of the Start Network to Set Cell Size. Frontiers in Cell and Developmental Biology 5. doi: 10.3389/fcell.2017.00057
Deniz Ö, Flores O, Aldea M, Soler-López M, Orozco M (2016) Nucleosome architecture throughout the cell cycle. Scientific Reports 6: 19729. doi: 10.1038/srep19729
Georgieva MV, Yahya G, Codó L, Ortiz R, Teixidó L, …, Gelpí JL, Gallego C, Orozco M, Aldea. (2015). Inntags: small self-structured epitopes for innocuous protein tagging. Nature Methods 12: 955–958. doi: 10.1038/nmeth.3556
Yahya G, Parisi E, Flores A, Gallego C, Aldea M. (2014). A Whi7-anchored loop controls de G1 Cdk-cyclin complex at Start. Molecular Cell 53: 115-126. doi: 10.1016/j.molcel.2013.11.015
Pedraza N, Ortiz R, Cornado A, Llobet A, Aldea M, Gallego C (2014) KIS, a kinase associated with microtubule regulators, enhances translation of AMPA receptors and stimulates dendritic spine remodeling. J Neurosci 34: 13988–97. doi: 10.1523/JNEUROSCI.1573-14.2014
Menoyo S, Ricco N, Bru S, Hernández-Ortega S, Escoté X, Aldea M, Clotet J. (2013). Phosphate-activated CDK stabilizes G1 cyclin to trigger cell cycle entry. Molecular and Cellular Biology 33: 1273-84. doi: 10.1128/MCB.01556-12
Ferrezuelo F, Colomina N, Palmisano A, Garí E, Gallego C, Csikasz-Nagy A, Aldea M. (2012). The critical size is set at a single-cell level by growth rate to attain homeostasis and adaptation. Nature Communications 3: 1012 p1-11. doi: 10.1038/ncomms2015
Fernández RMH, Ruiz-Miró M, Dolcet X, Aldea M, Garí E (2011) Cyclin D1 interacts and collaborates with Ral GTPases enhancing cell detachment and motility. Oncogene 30: 1936–46. doi: 10.1038/onc.2010.577
Ruiz-Miro M, Colomina N, Fernandez RMH, Gari E, Gallego C, Aldea M (2011) Translokin (Cep57) interacts with cyclin D1 and prevents its nuclear accumulation in quiescent fibroblasts. Traffic 12: 549–62. doi: 10.1111/j.1600-0854.2011.01176.x
Ferrezuelo F, Colomina N, Futcher B, Aldea M. (2010) The transcriptional network activated by Cln3 cyclin at the G1-to-S transition of the yeast cell cycle. Genome Biology 11: R67 p1-18. doi: 10.1186/gb-2010-11-6-r67
Cardus A, Panizo S, Encinas M, Dolcet X, Gallego C, Aldea M, Fernández E, Valdivielso JM (2009) 1,25-Dihydroxyvitamin D(3) regulates VEGF production through a vitamin D response element in the VEGF promoter. Atherosclerosis 204: 85–9. doi: 10.1016/j.atherosclerosis.2008.08.020
Ferrezuelo F, Aldea M, Futcher B (2009) Bck2 is a phase-independent activator of cell cycle-regulated genes in yeast. Cell Cycle 8: 239–52. doi: 10.4161/cc.8.2.7543
Pedraza N, Rafel M, Navarro I, Encinas M, Aldea M, Gallego C (2009) Mixed lineage kinase phosphorylates transcription factor E47 and inhibits TrkB expression to link neuronal death and survival pathways. Journal of Biological Chemistry 284: 32980–8. doi: 10.1074/jbc.M109.038729
Cambray S, Pedraza N, Rafel M, Gari E, Aldea M, Gallego C (2009) Protein kinase KIS localizes to RNA granules and enhances local translation. Molecular and Cellular Biology 29: 726–35. doi: 10.1128/MCB.01180-08
Colomina N, Ferrezuelo F, Verges E, Aldea M, Gari E (2009) Whi3 regulates morphogenesis in budding yeast by enhancing Cdk functions in apical growth. Cell Cycle 8: 1912–20. doi: 10.4161/cc.8.12.8740
Colomina N, Ferrezuelo F, Wang H, Aldea M, Gari E (2008) Whi3, a developmental regulator of budding yeast, binds a large set of mRNAs functionally related to the endoplasmic reticulum. Journal of Biological Chemistry 283: 28670–9. doi: 10.1074/jbc.M804604200
Aldea M, Gari E, Colomina N (2007) Control of cell cycle and cell growth by molecular chaperones. Cell Cycle 6: 2599–603. doi: 10.4161/cc.6.21.4920
Vergés E, Colomina N, Garí E, Gallego C, Aldea M. (2007). Cyclin Cln3 is retained at the ER and released by the J-chaperone Ydj1 in late G1 to trigger cell cycle entry. Molecular Cell 26: 649-662. doi: 10.1016/j.molcel.2007.04.023
Cardús A, Parisi E, Gallego C, Aldea M, Fernández E, Valdivielso JM (2006) 1,25-Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney Int 69: 1377–84. doi: 10.1038/sj.ki.5000304
Clotet J, Escoté X, Adrover MA, Garí E, Aldea M, De Nadal E, Posas F. (2006). Phosphorylation of Hsl1 by Hog1 leads to a G2 arrest essential for cell survival at high osmolarity. The EMBO Journal 25: 2338-2346. doi: 10.1038/sj.emboj.7601095
Liu YH, Encinas M, Comella JX, Aldea M, Gallego C (2004) Basic helix-loop-helix proteins bind to TrkB and p21(Cip1) promoters linking differentiation and cell cycle arrest in neuroblastoma cells. Molecular and Cellular Biology 24: 2662–72. doi: 10.1128/MCB.24.7.2662-2672.2004
Wang H, Garí E, Vergés E, Gallego C, Aldea M. (2004). Recruitment of Cdc28 by Whi3 restricts nuclear accumulation of the G1 cyclin-Cdk complex to late G1. The EMBO Journal 23: 180-190. doi: 10.1038/sj.emboj.7600022
Colomina N, Liu YH, Aldea M, Gari E (2003) TOR regulates the subcellular localization of Ime1, a transcriptional activator of meiotic development in budding yeast. Molecular and Cellular Biology 23: 7415–24. doi: 10.1128/MCB.23.20.7415-7424.2003
Ferrezuelo F, Steiner B, Aldea M, Futcher B (2002) Biogenesis of yeast telomerase depends on the importin Mtr10. Molecular and Cellular Biology 22: 6046–55. doi: 10.1128/MCB.22.17.6046-6055.2002
Cardus A, Parisi E, Muray S, Gallego C, Marco MP, Aldea M, Fernández E (2002) Calcitriol stimulates vascular smooth muscle cells (VSMCs) proliferation. J Am Soc Nephrol 13: 197A
Belli G, Gari E, Aldea M, Herrero E (2001) Osmotic stress causes a G(1) cell cycle delay and downregulation of Cln3/Cdc28 activity in Saccharomyces cerevisiae. Molecular Microbiology 39: 1022–35. doi: 10.1046/j.1365-2958.2001.02297.x
Garí E, Volpe T, Wang H, Gallego C, Futcher B, Aldea M. (2001). Whi3 binds the mRNA of the G1 cyclin CLN3 to modulate cell fate in budding yeast. Genes & Development 15: 2803-2808. doi: 10.1101/gad.203501
Colomina N, Garí E, Gallego C, Herrero E, Aldea M. (1999). G1 cyclins block the Ime1 pathway to make mitosis and meiosis incompatible in budding yeast. The EMBO Journal 18: 320-329. doi: 10.1093/emboj/18.2.320
Clotet J, Gari E, Aldea M, Arino J (1999) The yeast Ser/Thr phosphatases Sit4 and Ppz1 play opposite roles in regulation of the cell cycle. Molecular and Cellular Biology 19: 2408–15. doi: 10.1128/MCB.19.3.2408
Belli G, Gari E, Piedrafita L, Aldea N, Herrero E (1998) An activator/repressor dual system allows tight tetracycline-regulated gene expression in budding yeast. Nucleic Acids Research 26: 942–7. doi: 10.1093/nar/26.4.942
Wang RF, O’Hara EB, Aldea M, Bargmann CI, Gromley H, Kushner SR (1998) Escherichia coli mrsC is an allele of hflB, encoding a membrane-associated ATPase and protease that is required for mRNA decay. J Bacteriol 180: 1929–38. doi: 10.1128/JB.180.7.1929-1938.1998
Colomina N, Aldea M (1998) Lumi-imagine! direct quantification of chemiluminescent signals Biochemica 1: 21
Belli G, Gari E, Aldea M, Herrero E (1998) Functional analysis of yeast essential genes using a promoter-substitution cassette and the tetracycline-regulatable dual expression system. Yeast 14: 1127–38. doi: 10.1002/(SICI)1097-0061(19980915)14:12<1127::AID-YEA300>3.0.CO;2-%23
Gari E, Piedrafita L, Aldea M, Herrero E (1997) A set of vectors with a tetracycline-regulatable promoter system for modulated gene expression in Saccharomyces cerevisiae. Yeast 13: 837–48. doi: 10.1002/(SICI)1097-0061(199707)13:9<837::AID-YEA145>3.0.CO;2-T
Nadal A, Jares P, Cazorla M, Fernandez PL, Sanjuan X, Hernandez L, Pinyol M, Aldea M, Mallofre C, Muntane J, Traserra J, Campo E, Cardesa A (1997) p21(WAF1/Cip1) expression is associated with cell differentiation but not with p53 mutations in squamous cell carcinomas of the larynx. The Journal of Pathology 183: 156–63. doi: 10.1002/(SICI)1096-9896(199710)183:2<156::AID-PATH908>3.0.CO;2-O
Casas C, Aldea M, Espinet C, Gallego C, Gil R, Herrero E (1997) The AFT1 transcriptional factor is differentially required for expression of high-affinity iron uptake genes in Saccharomyces cerevisiae. Yeast 13: 621–37. doi: 10.1002/(SICI)1097-0061(19970615)13:7<621::AID-YEA121>3.0.CO;2-U
Gallego C, Garí E, Colomina N, Herrero E, Aldea M. (1997). The Cln3 cyclin is downregulated by translational repression and degradation during the G1 arrest caused by nitrogen deprivation in budding yeast. The EMBO Journal 16: 7196-7206. doi: 10.1093/emboj/16.23.7196
Dujon B, Albermann K, Aldea M, Alexandraki D, Ansorge W, et al. (1997). The nucleotide sequence of Saccharomyces cerevisiae chromosome XV. Nature 387(Suppl.): 98-102. doi: 10.1038/387s098
Gamo FJ, Lafuente MJ, Casamayor A, Arino J, Aldea M, Casas C, Herrero E, Gancedo C (1996) Analysis of the DNA sequence of a 15.5 kbp fragment near the left telomere of chromosome XV from Saccharomyces cerevisiae reveals a putative sugar transporter, a carboxypeptidase homologue and two new open reading frames. Yeast 12: 709–14. doi: 10.1002/(SICI)1097-0061(19960615)12:7<709::AID-YEA957>3.0.CO;2-1
Aldea M, Piedrafita L, Casas C, Casamayor A, Khalid H, Balcells L, Arino J, Herrero E (1996) Sequence analysis of a 12.8 kbp fragment of the left arm of yeast chromosome XV containing a putative 6-phosphofructo-2-kinase gene, a gene for a possible glycolphospholipid-anchored surface protein and six other open reading frames. Yeast 12 (sup): 1053–8. doi: 10.1002/(SICI)1097-0061(199609)12:10B<1053::AID-YEA993>3.0.CO;2-S
Casamayor A, Khalid H, Balcells L, Aldea M, Casas C, Herrero E, Gamo FJ, Lafuente MJ, Gancedo C, Arino J (1996) Sequence analysis of a 13.4 kbp fragment from the left arm of chromosome XV reveals a malate dehydrogenase gene, a putative Ser/Thr protein kinase, the ribosomal L25 gene and four new open reading frames. Yeast 12 (sup): 1013–20. doi: 10.1002/(SICI)1097-0061(199609)12:10B<1013::AID-YEA980>3.0.CO;2-5
Espinet C, Delatorre MA, Aldea M, Herrero E (1995) An efficient method to isolate yeast genes causing overexpression mediated growth arrest. Yeast 11: 25–32. doi: 10.1002/yea.320110104
Casamayor A, Aldea M, Casas C, Herrero E, Gamo FJ, Lafuente MJ, Gancedo C, Arino J (1995) DNA-sequence analysis of a 13 kbp fragment of the left arm of yeast chromosome -XV containing 7 new open reading frames. Yeast 11: 1281–8. doi: 10.1002/yea.320111308
Casas C, Aldea M, Casamayor A, Lafuente MJ, Gamo FJ, Gancedo C, Arino J, Herrero E (1995) Sequence analysis of a 9873 bp fragment of the left arm of yeast chromosome XV that contains the ARG8 and CDC33 genes, a putative riboflavin synthase beta-chain gene, and 4 new open reading frames. Yeast 11: 1061–7. doi: 10.1002/yea.320111107
Gan K, Sankaran K, Williams MG, Aldea M, Rudd KE, Kushner SR, Wu HC (1995) The umpA gene of Escherichia coli encodes phosphatidylglycerol:prolipoprotein diacylglyceryl transferase (lgt) and regulates thymidylate synthase levels through translational coupling. Journal of Bacteriology 177: 1879–982. doi: 10.1128/jb.177.7.1879-1882.1995
Garrido T, Aldea M (1994) Expresión de genes heterólogos en bacterias. Avances en Ingeniería Genética. Vicente, M. (Ed.). CSIC. Madrid
Comella JX, Sanzrodriguez C, Aldea M, Esquerda JE (1994) Skeletal muscle-derived trophic factors prevent motoneurons from entering an active cell-death program in vitro. J Neurosci 14: 2674–86. doi: 10.1523/JNEUROSCI.14-05-02674.1994
Aldea M, Garrido T, Tormo A (1993) Gearbox gene expression and growth rate. World Journal of Microbiology and Biotechnology 9: 414–20. doi: 10.1007/BF00328029
Garrido T, Sanchez M, Palacios P, Aldea M, Vicente M (1993) Transcription of ftsZ oscillates during the cell cycle of Escherichia coli. The EMBO Journal 12: 3957–65. doi: 10.1002/j.1460-2075.1993.tb06073.x
Vicente M, Aldea M, Sacristán A, Rohde C, Weihs V, Kracht M, van Asma F, Kampert E, Hughes V, Jones C (1992) A standardized format for handling data on plasmids, viruses and transposons: The PVT database format. World Journal of Microbiology and Biotechnology 8: 516–26. doi: 10.1007/BF01201952
Vicente M, Palacios P, Dopazo A, Garrido T, Pla J, Aldea M (1991) On the chronology and topography of bacterial cell division. Research in Microbiology 142: 253–7. doi: 10.1016/0923-2508(91)90038-C
Pla J, Sanchez M, Palacios P, Vicente M, Aldea M (1991) Preferential cytoplasmic location of Ftsz, a protein essential for Escherichia coli septation. Molecular Microbiology 5: 1681–6. doi: 10.1111/j.1365-2958.1991.tb01915.x
Vicente M, Kushner SR, Garrido T, Aldea M (1991) The role of the gearbox in the transcription of essential genes. Molecular Microbiology 5: 2085–91. doi: 10.1111/j.1365-2958.1991.tb02137.x
Aldea M, Garrido T, Pla J, Vicente M (1990) Division genes in Escherichia coli are expressed coordinately to cell septum requirements by gearbox promoters. The Embo Journal 9: 3787–94. doi: 10.1002/j.1460-2075.1990.tb07592.x
Aldea M, Garrido T, Hernandez-Chico C, Vicente M, Kushner SR (1989) Induction of a growth-phase-dependent promoter triggers transcription of bolA, an Escherichia coli morphogene. The EMBO Journal 8: 3923–31. doi: 10.1002/j.1460-2075.1989.tb08573.x
Hamilton CM, Aldea M, Washburn BK, Babitzke P, Kushner SR (1989) New method for generating deletions and gene replacements in Escherichia coli. Journal of Bacteriology 171: 4617–22. doi: 10.1128/jb.171.9.4617-4622.1989
Aldea M, Kushner SR (1988) CLONING – a microcomputer program for cloning simulations. Gene 65: 111–6. doi: 10.1016/0378-1119(88)90422-2
Aldea M, Maples VF, Kushner SR (1988) Generation of a detailed physical and genetic map of the ilv-metE-udp region of the Escherichia coli chromosome. Journal of Molecular Biology 200: 427–38. doi: 10.1016/0022-2836(88)90533-5
Aldea M, Kushner SR (1988) Instructions for the CLONING program. Gene 65: 117–22. doi: 10.1016/0378-1119(88)90423-4
Aldea M, Hernandez-Chico C, de la Campa AG, Kushner SR, Vicente M (1988) Identification, cloning, and expression of bolA, an ftsZ-dependent morphogene of Escherichia coli. Journal of Bacteriology 170: 5169–76. doi: 10.1128/jb.170.11.5169-5176.1988
de la Campa AG, Aldea M, Hernández-Chico C, Tormo A, Vicente M (1988) Segregation of elongation potential in Escherichia coli mediated by the wee genetic system. Current Microbiology 17: 315–9. doi: 10.1007/BF01570871
Aldea M, Claverie-Martin F, Diaz-Torres MR, Kushner SR (1988) Transcript mapping using S35 DNA probes, trichloroacetate solvent and dideoxy sequencing ladders – a rapid method for identification of transcriptional start points. Gene 65: 101–10. doi: 10.1016/0378-1119(88)90421-0
Dopazo A, Tormo A, Aldea M, Vicente M (1987) Structural inhibition and reactivation of Escherichia coli septation by elements of the SOS and TER pathways. Journal of Bacteriology 169: 1772–6. doi: 10.1128/jb.169.4.1772-1776.1987
Tormo A, Ayala JA, de Pedro MA, Aldea M, Vicente M (1986) Interaction of FtsA and PBP3 proteins in the Escherichia coli septum. Journal of Bacteriology 166: 985–92. doi: 10.1128/jb.166.3.985-992.1986
de la Campa AG, Tormo A, Martinez-Salas E, Aldea M, Vicente M (1985) Cell length in a wee dnaA mutant of Escherichia coli. Journal of Bacteriology 164. doi: 10.1128/jb.164.1.487-489.1985
Tormo A, Dopazo A, Delacampa AG, Aldea M, Vicente M (1985) Coupling between DNA replication and cell division mediated by the FtsA protein in Escherichia coli, a pathway independent of the SOS response, the TER pathway. Journal of Bacteriology 164: 950–4. doi: 10.1128/jb.164.2.950-953.1985
Herrero E, Aldea M, Ros J, Monfort JM, Guerrero R (1982) Regulación de la division celular en Salmonella typhimurium. En Jiménez-Sánchez, A. and R. Guerrero (Eds.), pp 265-283. Genética Molecular Bacteriana. Reverté. Barcelona
Herrero E, Aldea M, Guerrero R (1982) Ciclo celular en bacterias. I. Aspectos moleculares. En Jiménez-Sánchez, A. y R. Guerrero (Eds.), pp 173-207. Genética Molecular Bacteriana. Reverté. Barcelona
Aldea M, Herrero E, Trueba FJ (1982) Constancy of diameter through the cell cycle of Salmonella typhimurium LT2. Current Microbiology 7: 165–8. doi: 10.1007/BF01568969
Aldea M, Herrero E, Esteve MI, Guerrero R (1980) Surface density of major outer-membrane proteins in Salmonella-typhimurium in different growth-conditions. J Gen Microbiol 120. doi: 10.1099/00221287-120-2-355
Project funding
Protein cluster dynamics and network channeling
MICINN PID2019-109638GB-I00, 2020-2022
Proyecto PID2019-109638GB-I00 financiado por:

Antiprions: a screen for prion mutants interfering with endogenous prion aggregation
AEI (EXPLORA), BIO2017-91613-EXP, 2019-2020
Proyecto BIO2017-91613-EXP financiado por:

Proteotoxic aggregates, chaperome alterations and cell proliferation decline during cell aging
MINECO, BFU2016-80234-R, 2017-2019
Proyecto BFU2016-80234-R financiado por:

Prions and aggregons as inhibitors of Start: a path to cell ageing
MINECO. BFU2013-47710-R, 2014-2016
EpiTag: Development of new peptidic tags
MICINN IPT-010000-2010-019, 2011-2013
Molecular competition and cell size control
MICINN BFU2010-20205/BFS, 2011-2013
Vacancies/Jobs
We continuously hold PhD student positions granted by the Spanish Ministry of Science and Innovation. Applications will be considered ASAP.
Contact