Lab presentation
Our group is interested in the processes of proliferation and differentiation of neuronal precursors and in the consequences of the malfunction of these mechanisms. During our trajectory, we have used different models to study this process, for example, the precursors of the granular cells of the rat cerebellum. Granular cells are very abundant, and in humans, they give rise to Medulloblastomas, tumours of the cerebellum that develop at an early age. Using this model, we described the regulation that BMPs make on Sonic Hedgehog (Shh), one of the genes whose mutation gives rise to Medulloblastomas, in addition, also using this model we described important characteristics of Shh pathway, including the crucial presence of PKA and adenylate cyclases in the primary cilium of these cells. In an attempt to have a physiological model to study neuronal proliferation and differentiation, we adopted chick embryo “in ovo” model. This has allowed us to study basic features of developing neuroepithelia, such as, cell polarity or invasion of the surrounding mesenchyme by cells carrying oncogenes. At present, our interest remains the same as at the beginning, finding the mechanisms that make a cell multiply and, when the time comes, differentiate into a complex structure such is an adult neuron. In this sense, we are very interested in molecules such as N-cadherin and b-catenin, which are the basis of the mechanisms that regulate adhesion and neuronal polarity both in the proliferative and differentiation periods.
Projects
General project hypothesis
The hypothesis of the project proposes that during the nervous system development β-catenin carries out two sequential but interconnected activities. During NSCs proliferation, β-catenin activity is essential for the formation and maintenance of the AJs and especially for N-cadherin related adhesion and polarity. During this period, β-catenin is docked at the adherens junctions (AJs), preventing this way its transcriptional activity. We propose that β-catenin develops simultaneous tumour promoting and tumour suppressor activities and that the tumour suppressor activity, relays on its capacity to promote N-cadherin maturation. We also propose that when neurogenesis begins newly synthetized β-catenin is redirected towards the nucleus, where it activates Tcf dependent transcription. However, in contrast to the stemness promoting effect that it develops in NSCs, during neurogenesis β-catenin dependent transcription is crucial for neural differentiation.
Specific project objectives
β-cathenin regulation of apical complex adherens junctions (AC/AJs)
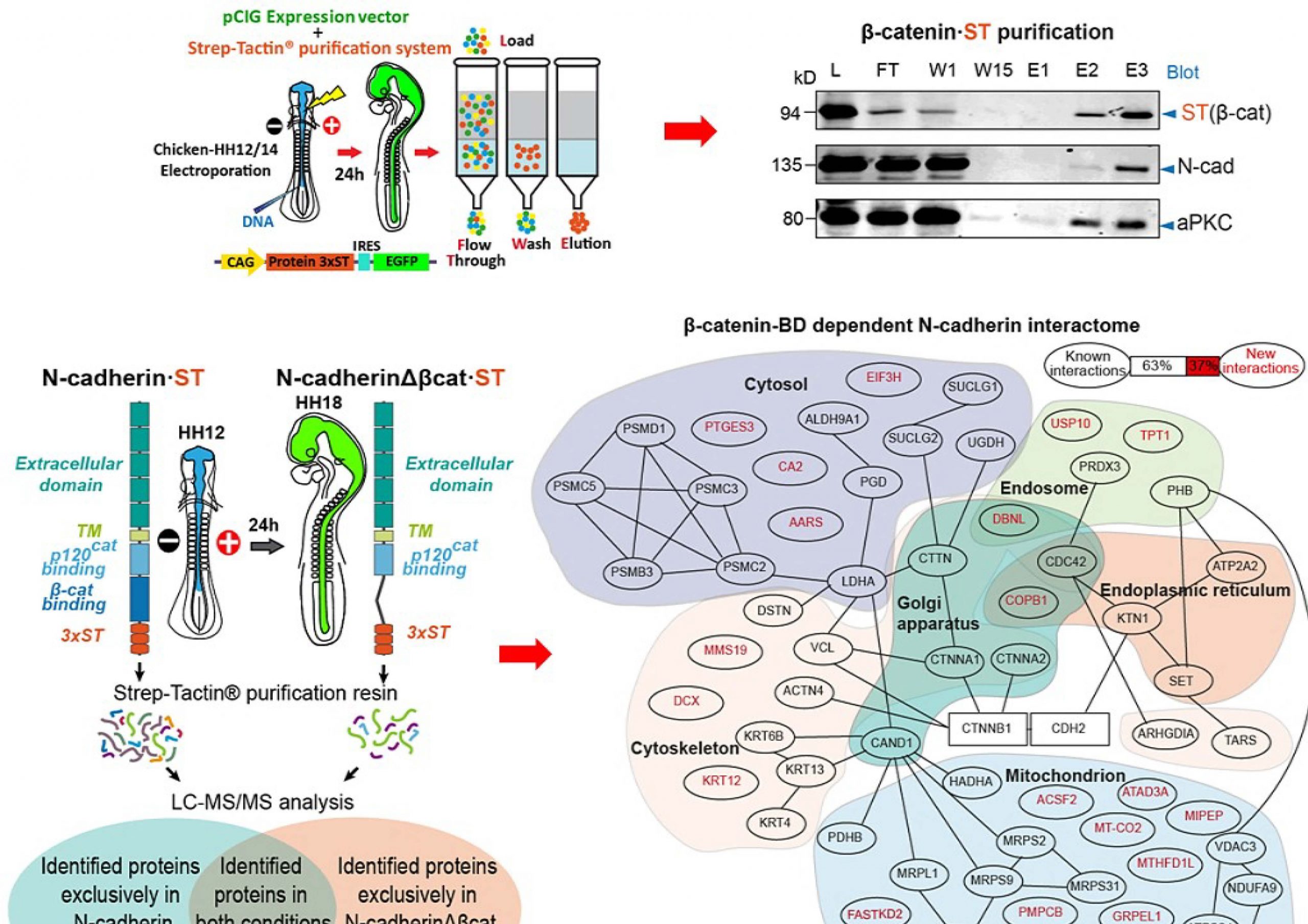
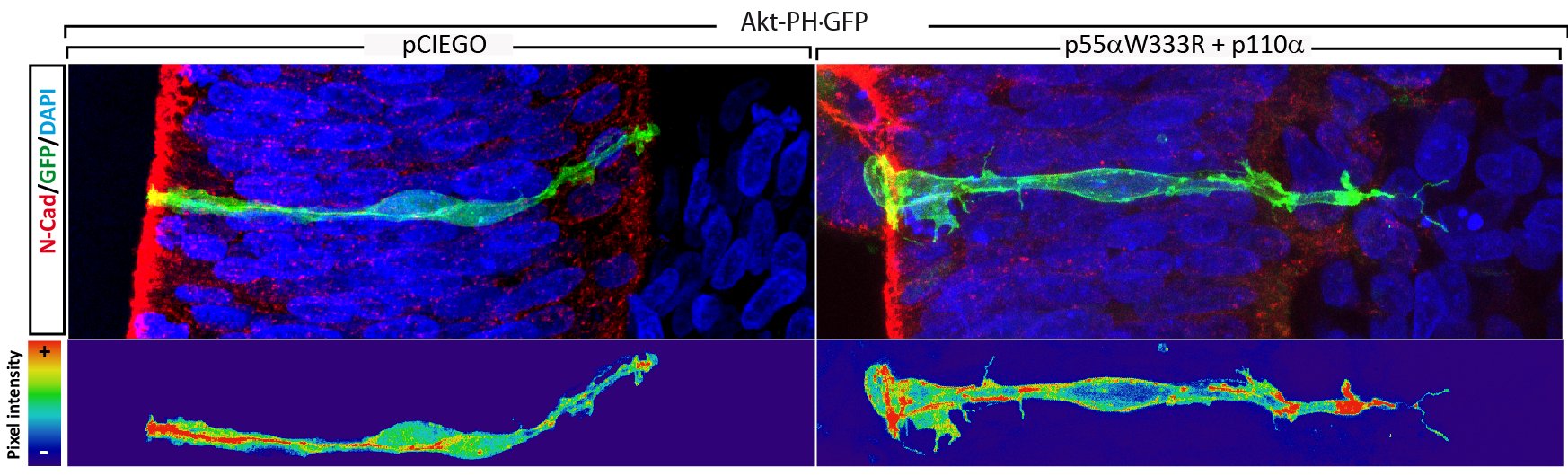
We have learned a lot about on how β-catenin contributes to AJs, especially in what concerns the metabolism of N-cadherin; however, we need to learn more about a complex that represents the core of the apical adhesion system in the developing nervous system. New results as the interaction with vinculin and its effects on interkinetic nuclear migration (INM) and cell cycle, told as that the regulation carried out by β-catenin is more relevant an also more complex than we thought at first. Therefore, we propose to study the role played by the different proteins associated with the N-Cadherin/β-Catenin complex on the formation of the AC/AJs, how they regulate apical and nuclear pools of β-Catenin and the effects that cause their deregulation on polarity, proliferation and differentiation of neuroblasts. Devoting special interest in the relevance of these AC/AJs associated proteins on β-Catenin dependent tumour progression.
Role of β-catenin during neurogenesis.
We discovered that Tcf-dependent transcription becomes re-activated during neurogenesis quite long ago, so, we have developed a new set of molecular tools to dissect all the events occurring during this process. Therefore, we propose to study in detail the time sequence and the molecular elements intervening in such process. Moreover, we already know what happens if an anomalous Tcf transcriptional activity occurs during NSCs proliferation, but we did not know what happens if it occurs during neural differentiation. I mean, we will study the consequences of the failure of what could be called “the β-catenin second life”.
Lab people

Sebastian Pons Fuxa
Principal investigator
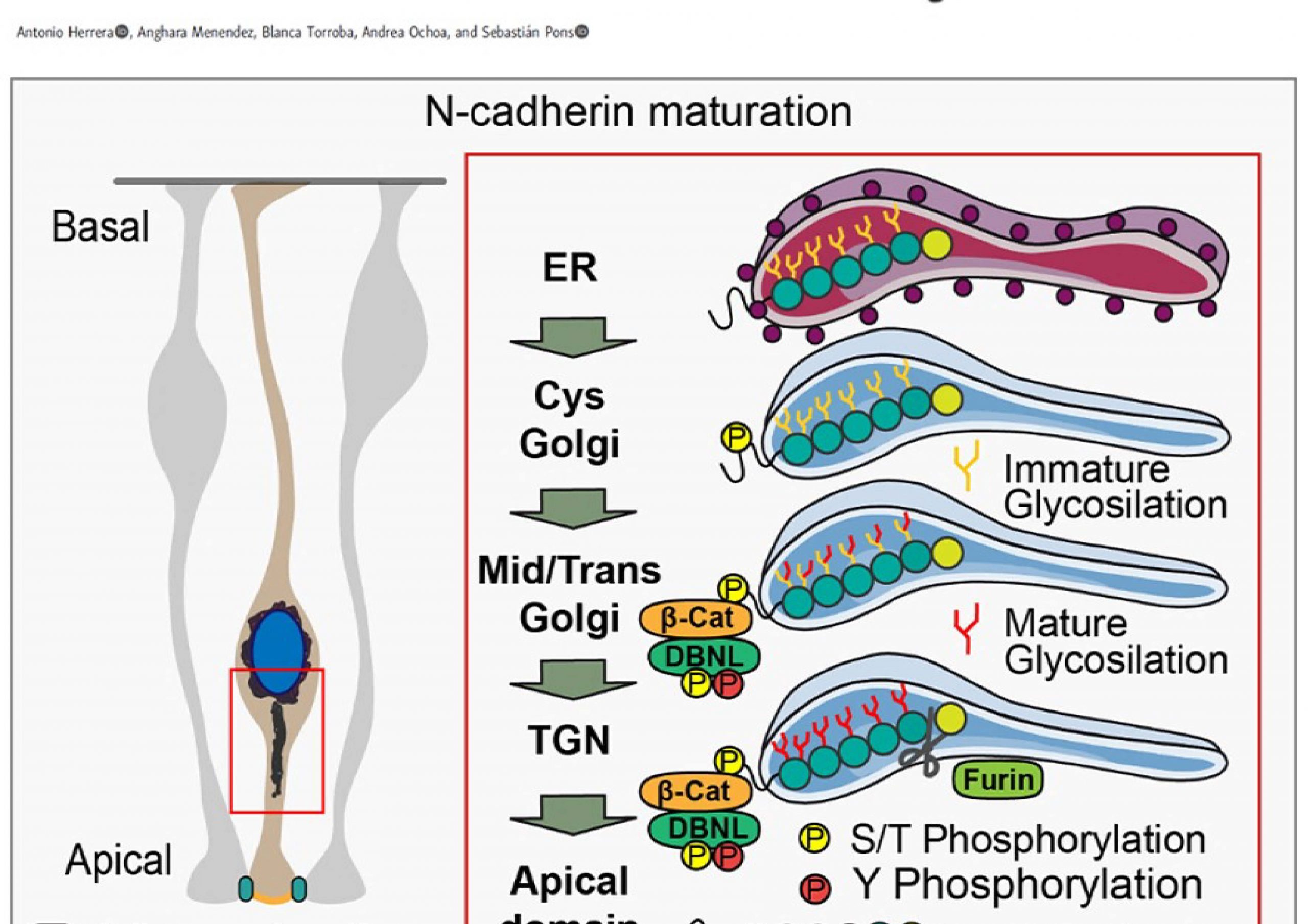
(1985-1989) Degree in Biology at the University of the Balearic Islands. (1990-1993) Doctoral thesis at the Cajal Institute directed by Dr Ignacio Torres Alemán. (1994-1997) Post-doc in Dr Morris White’s lab at Joslin Diabetes Center (Harvard Medical School), there, we described a new subunit of PI3K (PIK3R3). (1998) Back to Cajal Institute with Dr. Torres Alemán, where I studied the neuroprotective activity of IGF-1. (2001) Gained a position of Científico titular del CSIC at the IIBB (Barcelona), to study the regulation of proliferation and differentiation of embryonic neural stem cells. (2014) Moved to the IBMB (Barcelona). Although our main interest has always been studying the proliferation and differentiation process, our research led us to study the activity of molecules such as Sonic Hedgehog, BMPs or Wnt factors. Thus, we studied the relevance on Shh pathway of Adenylate Cyclases, Heterotrimeric G proteins or PKA (Barzi et al 2010, Barzi et al 2011, Vuolo et al 2015). Similarly, we used chicken embryos to generate a model of Wnt-type Medulloblastoma (Herrera et al, 2014), where we studied the early events occurring in this tumor, or the role of PI3K in intraepithelial positioning (Torroba et al. 2018). In this line, we have recently shown that β-catenin binds to pro-N-cadherin early in the secretory pathway promoting its maturation and its delivery to the adherens junctions (Herrera et al 2021).

Anghara Menéndez

Carlota Ramos Corral
Past students
Antonio Herrera
Andrea Ochoa
Laura Vuolo
Maria Blanca Torroba
María Estefanell Alós
Ruben Alvarez
Mercedes Barzi
Jordi Berenguer
Selected publications
Ochoa A, Herrera A, Menendez A, Estefanell M, Ramos C, Pons S.
Vinculin is required for interkinetic nuclear migration (INM) and cell cycle progression.
Journal of Cell Biology 223 (1): e202106169 (2024)
Herrera A, Menendez A, Ochoa A, Bardia L, Columbelli J and Pons S.
Neurogenesis redirects β-catenin from adherens junctions to the nucleus to promote axonal growth.
Development 150: (2023)
Herrera A, Menendez A, Torroba B, Ochoa A and Pons S.
Dbnl and β-catenin promote pro-N-cadherin processing to maintain apico-basal polarity.
J Cell Biol 7 June 2021; 220 (6): e202007055
Torroba B, Herrera A, Menendez A and Pons S.
PI3K regulates intraepithelial cell positioning through Rho GTP-ases in the developing neural tube.
Developmental Biology 436:42-54 (2018)
Rabadan MA, Herrera A, Fanlo L, Usieto S, Carmona C, Barriga E, Mayor R, Pons S, and Marti E.
Delamination of neural crest cells requires transient and reversible Wnt inhibition mediated by DACT1/2.
Development 143:2194-205 (2016)
Vuolo L, Herrera A, Torroba B, Menendez A and Pons S.
Ciliary Adenilyl Cyclases control Hedgehog pathway.
J Cell Sci 128:2928-37 (2015)
Díaz de Greñu B, García-Calvo J, Cuevas J, García-Herbosa G, García B, Busto N, Ibeas S, Torroba T, Torroba B, Herrera A and Pons S
Chemical speciation of MeHg+ and Hg 2+ in aqueous solution and HEK cells nuclei by means of DNA interacting fluorogenic probes.
Chem Sci 6:3757-3764 (2015)
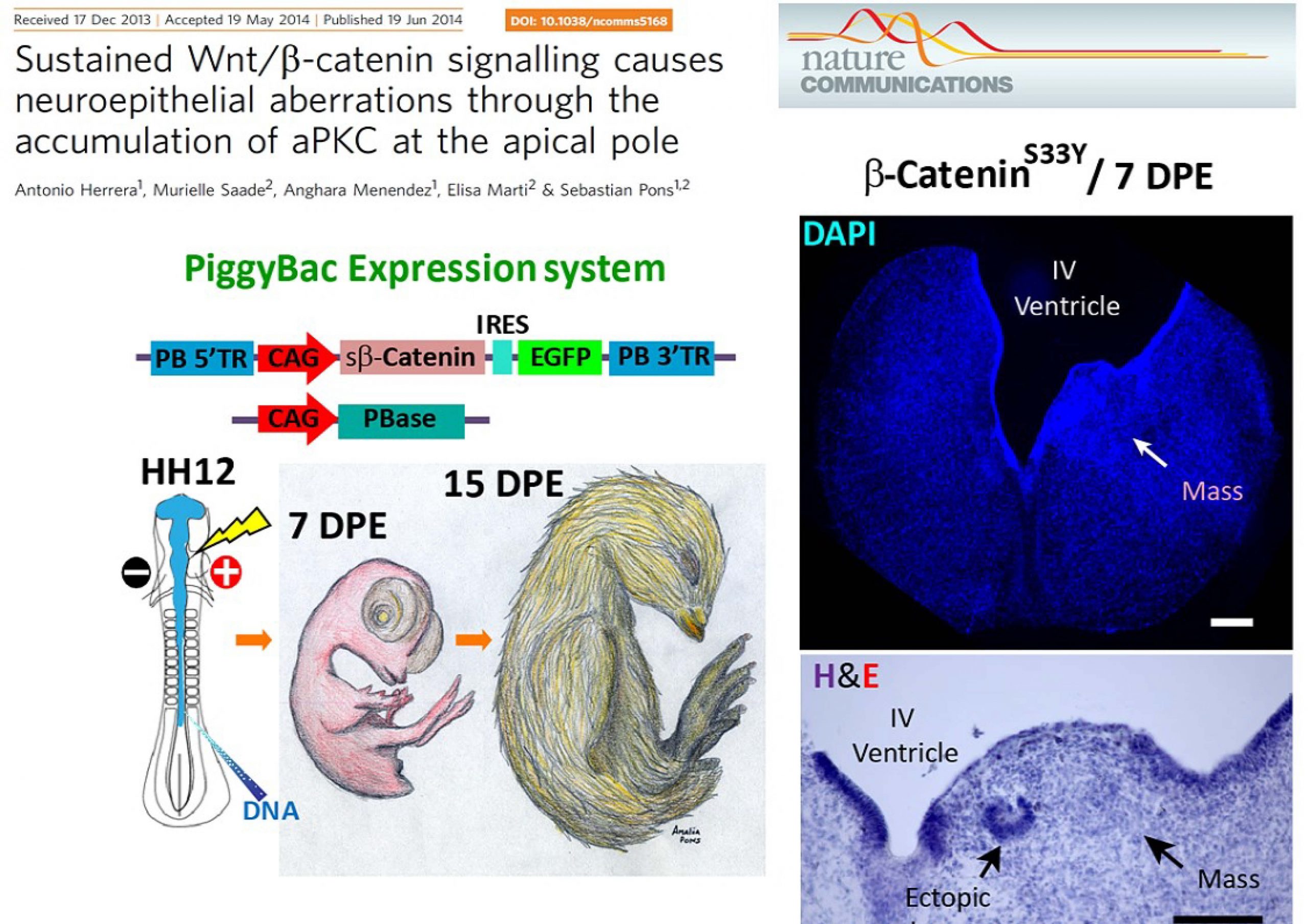
Herrera A, Saade M, Menendez A, Marti E and Pons S.
Sustained Wnt/β-Catenin signaling causes neuroepithelial aberrations through the accumulation of aPKC at the apical pole.
Nat Commun 5:4168-81(2014)
Berenguer J, Herrera A, Vuolo L, Torroba B, Llorens F, Sumoy L and Pons S.
Mir-22 regulates cell-cycle length in Cerebellar Granular Neuron Precursors.
Mol Cell Biol. 33(14):2706-2717 (2013)
Barzi M, Kostrz D, Menendez A and Pons S.
Sonic Hedgehog induced proliferation requires specific G-alpha-inhibitory proteins.
J Biol Chem. 286(10):8067–8074 (2011).
Barzi M, Berenguer J, Menendez A, Alvarez-Rodriguez R and Pons S.
Sonic-hedgehog-mediated proliferation requires the localization of PKA to the cilium base.
J Cell Sci. 123:62-69 (2010)
Álvarez-Rodríguez R, and Pons S.
Expression of the proneural gene encoding Mash1 suppresses MYCN mitotic activity.
J Cell Sci. 122:595-9. (2009)
Alvarez-Rodriguez R, Barzi M, Berenguer J, Pons S.
BMP2 opposes SHH mediated proliferation in cerebellar granule cells through a TIEG-1 based regulation of Nmyc.
J Biol Chem 282: 37170-80 (2007)
Rios I, Alvarez-Rodriguez R, Marti E, Pons S.
Bmp2 antagonizes sonic hedgehog-mediated proliferation of cerebellar granule neurones through Smad5 signaling.
Development. 131(13):3159-68. (2004)
All publications
Ochoa A, Herrera A, Menendez A, Estefanell M, Ramos C, Pons S.
Vinculin is required for interkinetic nuclear migration (INM) and cell cycle progression.
Journal of Cell Biology 223 (1): e202106169 (2024)
Herrera A, Menendez A, Ochoa A, Bardia L, Columbelli J and Pons S.
Neurogenesis redirects β-catenin from adherens junctions to the nucleus to promote axonal growth.
Development 150: (2023)
Dutto I, Gerhards J, Herrera A, Souckova O, Škopová V, Smak JA, Junza A, Yanes O, Boeckx C, Burkhalter MD, Zikánová M, Pons S, Philipp M, Lüders J and Stracker TH.
Pathway specific effects of ADSL deficiency on neurodevelopment
Elife 11, e70518 (2022)
Gradari S, Herrera A, Tezanos P, Fontán-Lozano A, Pons S, and Trejo JL.
The role of Smad2 in adult neuroplasticity as seen through hippocampal-dependent spatial learning/memory and neurogenesis.
Journal of Neuroscience 41: 6836-6849 (2021)
Herrera A, Menendez A, Torroba B, Ochoa A and Pons S.
Dbnl and β-catenin promote pro-N-cadherin processing to maintain apico-basal polarity.
J Cell Biol 7 June 2021; 220 (6): e202007055
Busto N, García-Calvoa J, Cuevas JV, Herrera A, Mergny JL, Pons S, Torroba T and García B.
Influence of core extension and side chain nature in targeting G-quadruplex structures with perylene monoimide derivatives.
Bioorganic Chemistry. 108:104660 (2021)
Le Dreau G, Escalona R, Fueyo R, Herrera A, Martinez JD, Usieto S, Menendez A, Pons S, Martinez-Balbas MA and Marti E
E proteins sharpen neurogenesis by modulating proneural bHLH transcription factors’ activity in an E-box-dependent manner.
eLife 2018; 7 (2018)
Torroba B, Herrera A, Menendez A and Pons S.
PI3K regulates intraepithelial cell positioning through Rho GTP-ases in the developing neural tube.
Developmental Biology 436:42-54 (2018)
Rabadan MA, Herrera A, Fanlo L, Usieto S, Carmona C, Barriga E, Mayor R, Pons S, and Marti E.
Delamination of neural crest cells requires transient and reversible Wnt inhibition mediated by DACT1/2.
Development 143:2194-205 (2016)
Vuolo L, Herrera A, Torroba B, Menendez A and Pons S.
Ciliary Adenilyl Cyclases control Hedgehog pathway.
J Cell Sci 128:2928-37 (2015)
Díaz de Greñu B, García-Calvo J, Cuevas J, García-Herbosa G, García B, Busto N, Ibeas S, Torroba T, Torroba B, Herrera A and Pons S
Chemical speciation of MeHg+ and Hg 2+ in aqueous solution and HEK cells nuclei by means of DNA interacting fluorogenic probes.
Chem Sci 6:3757-3764 (2015)
Herrera A, Saade M, Menendez A, Marti E and Pons S.
Sustained Wnt/β-Catenin signaling causes neuroepithelial aberrations through the accumulation of aPKC at the apical pole.
Nat Commun 5:4168-81(2014)
Miguez D, Gil-Guiñon E, Pons S and Marti E.
Smad2 and Smad3 cooperate and antagonize simultaneously in vertebrate neurogenesis.
J Cell Sci. 126(23):5335-43(2013)
Berenguer J, Herrera A, Vuolo L, Torroba B, Llorens F, Sumoy L and Pons S.
Mir-22 regulates cell-cycle length in Cerebellar Granular Neuron Precursors.
Mol Cell Biol. 33(14):2706-2717 (2013)
Rabadán MA, Cayuso J, Le Dréau G, Cruz C, Barzi M, Pons S, Briscoe J and Martí E.
Jagged2 controls the generation of motor neuron and oligodendrocyte progenitors in the ventral spinal cord.
Cell Death Differ. 192(2):209-19(2012)
Martı ED, Sanchez-Perez A, Trejo JL, Martin-Aldana JA, Cano Jaimez M, Pons S, Acosta Umanzor C, Menes L, MF. White and. Burks DJ.
IRS-2 Deficiency Impairs NMDA Receptor-Dependent Long-term Potentiation.
Cerebral Cortex. 22(8:1717 (2012)
Barzi M, Kostrz D, Menendez A and Pons S.
Sonic Hedgehog induced proliferation requires specific G-alpha-inhibitory proteins.
J Biol Chem. 286(10):8067–8074 (2011).
Barzi M, Berenguer J, Menendez A, Alvarez-Rodriguez R and Pons S.
Sonic-hedgehog-mediated proliferation requires the localization of PKA to the cilium base.
J Cell Sci. 123:62-69 (2010)
Álvarez-Rodríguez R, and Pons S.
Expression of the proneural gene encoding Mash1 suppresses MYCN mitotic activity.
J Cell Sci. 122:595-9. (2009)
Farina M, Campos F, Vendrell I, Berenguer J, Barzi M, Pons S, Suñol C. Probucol Increases Glutathione Peroxidase-1 Activity and Displays Long-Lasting Protection Against Methylmercury Toxicity in Cerebellar Granule Cells.
Toxicological Sciences 112 (2): 416-426 (2009)
Alvarez-Rodriguez R, Barzi M, Berenguer J, Pons S.
BMP2 opposes SHH mediated proliferation in cerebellar granule cells through a TIEG-1 based regulation of Nmyc.
J Biol Chem 282: 37170-80 (2007)
Rios I, Alvarez-Rodriguez R, Marti E, Pons S.
Bmp2 antagonizes sonic hedgehog-mediated proliferation of cerebellar granule neurones through Smad5 signaling.
Development. 131(13):3159-68. (2004)
Blaess S, Graus-Porta D, Belvindrah R, Radakovits R, Pons S, Littlewood-Evans A, Senften M, Guo H, Li Y, Miner JH, Reichardt LF, Muller U. Beta1-integrins are critical for cerebellar granule cell precursor proliferation.
J Neurosci. 24(13):3402-12 (2004)
Bortolozzi A, Amargos-Bosch M, Adell A, Diaz-Mataix L, Serrats J, Pons S, Artigas F. In vivo modulation of 5-hydroxytryptamine release in mouse prefrontal cortex by local 5-HT(2A) receptors: effect of antipsychotic drugs.
Eur J Neurosci. 18(5):1235-46. (2003)
Garcia-Galloway E, Arango C, Pons S, Torres-Aleman I. Glutamate excitotoxicity attenuates insulin-like growth factor-I prosurvival signaling.
Mol Cell Neurosci. 24(4):1027-37. 2003
Valverde AM, Arribas M, Mur C, Navarro P, Pons S, Cassard-Doulcier AM, Kahn CR, Benito M. Insulin-induced up-regulated uncoupling protein-1 expression is mediated by insulin receptor substrate 1 through the phosphatidylinositol 3-kinase/Akt signaling pathway in fetal brown adipocytes.
J Biol Chem. 278(12):10221-31 (2003)
Hansen HH, Azcoitia I, Pons S, Romero J, Garcia-Segura LM, Ramos JA, Hansen HS, Fernandez-Ruiz J. Blockade of cannabinoid CB(1) receptor function protects against in vivo disseminating brain damage following NMDA-induced excitotoxicity.
J Neurochem. 82(1):154-8 (2002)
Gonzales de la Vega A, Buño W, Pons S, Garcia-Calderat E, Garcia-Galloway E and Torres-Aleman I. Insulin-like growth factor I potentiantes kainate receptors through a phosphatidylinositol 3-kinase dependent pathway.
NeuroReport. 12(6):1293-6 (2001).
Pons, S., Trejo, J.L., Martinez-Morales JR., Martí E. Vitronectin regulates Sonic hedgehog activity in cerebellum development through CREB phosphorylation.
Development. 128(9):1481-92 (2001).
Valverde, A.M. Mur, C. Pons, S. Alvarez, A.M. White, M.F. Kahn, C.R. Benito, M. Y895IRS-I/GRB-2 association mediates the insulin signaling involved in IRS-1-deficient brown adipocytes mitogenesis.
Mol. Cell. Biol. 21(7):2269-89 (2001).
Trejo J.L. Pons S. Phosphatidylinositol 3-kinase regulatory subunits are differentially expressed during development of the cerebellum.
Neurobiology. 47(1): 39-50 (2001)
Pons, S. Torres-Alemán, I. IGF-I induces IkBa dephosphorylation through the Ser phosphatase Calcineurin (PP2B).
J.Biol.Chem. 275(49):38620-38625 (2000)
Pons S., Martí E. Sonic hedgehog synergizes with the extracellular matrix protein Vitronectin, to induce spinal motor neuron differentiation.
Development. 127: 333-342 (2000)
Schuppin GT, Pons S, Hugl S, Aiello LP, King GL, White M, Rhodes CJ. A specific increased expression of insulin receptor substrate 2 in pancreatic beta-cell lines is involved in mediating serum stimulated beta-cell growth.
Diabetes. 47(7): 1074-1085(1998)
Withers,DJ., Sanches,J., Towery, H., Burks, DJ., Jian-Ming, R., Previs, S., Zhang, Y., Bernal, D., Pons, S., Shulman, G.I., Bonner-Weir, S., White, MF. Disruption of IRS-2 causes Type 2 diabetes in mice.
Nature. 321: 900-904 (1998)
Valverde AM, Lorenzo M, Pons, S, White MF, Benito M. Insulin receptor substrate (IRS) proteins IRS-1 and IRS-2 differential signaling in the insulin/insulin-like growth factor-I pathways in fetal brown adipocytes.
Mol Endocrinol. 12(5):688-697 (1998).
Mothe I, Delahaye L, Filloux C, Pons S, White MF, Van Obberghen. Interaction of wild type and dominant-negative p55PIK regulatory subunit of phosphatidylinositol 3-kinase with Insulin-like growth factor-1 signaling proteins.
Mol Endocrinol 11(13):1911-1923 (1997)
Kerouz, N.J., Horsh, D., Pons, S., and Kahn, C.R. J . Differential regulation of insulin receptor substrates-1 and -2 (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in liver and muscle of the obese diabetic (OB/OB) mouse.
J Clin Invest. 100(12): 3164-3172 (1997).
Joyal, L.J., Burks, D., Pons, S., Matter, W.F., Vlahos, C.J., White, M.F and Sacks, D.B. Calmodulin activates Phosphatidylinositol 3-kinase.
J.Biol.Chem. 272: 28183-6 (1997)
Burks, D., Pons, S., Towery, H., Smith-Hall, J., Myers, M., Yenush, L., and White, MF. Heterologous PH domains do not couple IRS-1 to the insulin receptor.
J.Biol.Chem. 272: 27716-21 (1997)
Burfoot MS, Rogers NC, Watling D, Smith JM, Pons, S, Paonessaw G, Pellegrini S, White MF, Kerr IM. Janus kinase-dependent activation of Insulin receptor substrate 1 in response to interleukin-4, oncostatin M, and the interferons.
Biol. Chem. 272 (39): 24183-24190 (1997)
Uddin S, Sher DA, Alsayed Y, Pons, S, Colamonici OR, Fish EN, White MF, Platanias LC. Interaction of p59fyn with interferon-activated Jak kinases. Biochem Biophys Res Commun. 235(1):83-88 (1997)
Smith-Hall, J., Pons, S., Patti, M.E., Burks, D.J., Yenush, L., Sun, X.J., Kahn, C.R., and White, M.F. The 60-kDa insulin receptor substrate functions like an IRS-protein (pp60IRS3) in adipocytes.
Biochemistry. 36 (27) 8304-8310 (1997)
Sun, X.J., Pons, S., Wang, L.M., Zhang, Y., Yenush, L., Burks, D., Myers, M.G.,Jr., Glasheen,E., Copeland, N.G., Jenkins, N.A.,Pierce, J.H., and White, M.F. The IRS-2 gene on murine chromosome 8 encodes a unique signaling adapter for insulin and cytokine action.
Mol. Endocrinol. 11(2):251-262. (1997)
Sun, X.J., Pons, S., Asano, T., Myers, M.G.,Jr., Glasheen, E.M., and White, M.F. The fyn tyrosine kinase binds IRS-1 and forms a distinct signaling complex during insulin stimulation.
J.Biol.Chem. 271(18):10583-10587. (1996).
Pons, S., Asano, T., Glasheen, E.M., Miralpeix, M., Zhang, Y., Fisher, T.L., Myers, M.G.,Jr., Sun, X.J., and White, M.F. The structure and function of p55PIK reveals a new regulatory subunit for the phosphatidylinositol-3 kinase.
Mol.Cell Biol. 15(8):4453-4465. (1995)
Torres-Aleman, I., Pons, S., Arevalo, M.A. The insulin-like growth factor I system in the rat cerebellum: developmental regulation and role in neuronal survival and differentiation.
J.Neurosci.Research. 39(2):117-126 (1994)
Pons, S., Torres-Aleman. Estradiol modulates insulin-like growth factor I receptors and binding proteins in neurons from the hypothalamus.
Journal of Neuroendocrinology. 5(3):267-271 (1993).
Pons, S. I. Torres-Aleman. Basic fibroblast growth factor modulates insulin-like growth factor I, its receptor, and its binding proteins in hypothalamic cell cultures.
Endocrinology. 131, 2271-2278 (1992).
Torres-Alemán, I., Pons S., F.F. Santos-Benito. Survival of Purkinge cells in cerebelar culture is increased by Insulin Like Growth Factor I.
European. J. Neuroscience. 4:864-869 (1992)
Torres-Aleman, I., M.T. Rejas, S. Pons, L.M. Garcia-Segura. Estradiol promote cell chape changes and Glial Fibrilary Acid Potein in hypothalamic astrocites in vitro: a neuronal mediated effect.
Glia. 6:180-187 (1992)
Torres-Aleman, S. Pons, L.M. Garcia-Segura. Climbing fiber deafferentation reduces insulin-like growth factor I (IGF-I) content in cerebellum.
Brain Research. 564:348-351 (1991).
Pons, S., M.T. Rejas, I. Torres-Aleman. Ontogeny of Insulin-Like Growth Factor I (IGF-I), its receptor, and its binding proteins in the rat hypothalamus.
Developmental Brain Research. 62:169-175 .(1991)
Garcia-Segura, L.M, J. Perez, S. Pons, M.T. Rejas and I. Torres-Aleman. Localization of Insulin-Like Growth Factor I (IGF-I)-like immunoreactivity in the rat brain.
Brain Research. 560:167-174 (1991).
Pons, S., J.A. Lopez., C. Ramis., B. Planas., and R. Rial. “A new, precise, microcomputer based, rotometer”
Journal of Neuroscience Methods. 32:1955-1958 (1990)
Project funding
Project funding as principal investigator (10 years):
PID2020-116806GB-I00 MICIN (2021-2023)
b-Catenin controls the cell adhesion and differentiation of embryonary neural stem cells
Proyecto PID2020-116806GB-I00 financiado por:

BFU2017-83562-P MINECO (2018-2020)
The multiple actions of β-Catenin along the development of the nervous system and the consequences of its deregulation.
Proyecto BFU2017-83562-P financiado por:

BFU2014-53633-P MINECO (2015-2017)
Control of proliferation/differentiation in nervous system development.
BFU2011-24099 MCIN (2012-2014)
Mechanisms controlling the proliferation and differentiation processes during the nervous system development.
Vacancies/Jobs
If you are interested in joining the lab as postdoc or PhD student please send us your CV and cover letter: Sebastian Pons Fuxa (spfbmc@ibmb.csic.es).
Project gallery
No albums or photos uploaded yet.