Lab presentation
During animal development, the differentiation of cells, tissues and organs is tightly regulated through specific gene expression programs. Our group is particularly interested in studying the transcriptional and cell signaling mechanisms responsible for this control.
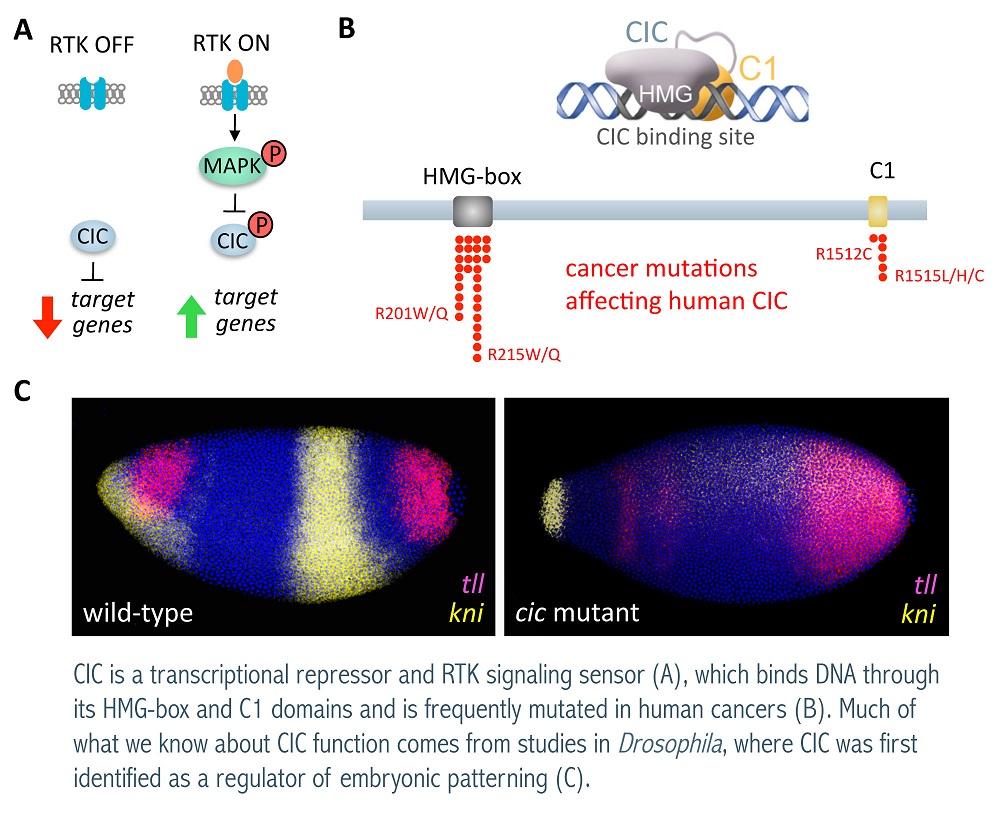
Using Drosophila as a model system, we have been dissecting the activities of repressor and co-repressor factors, as well as the responses induced by receptor tyrosine kinase (RTK) pathways during pattern formation and differentiation. One emerging theme has been the identification of the Capicua transcriptional repressor as a general sensor targeted by multiple RTK-initiated signals. Because the molecules and pathways that we study are conserved in evolution, our results have direct implications for human biology and disease.
To find out more about our lab, please visit also my ICREA website
Projects
Molecular analysis of Capicua, an RTK signaling sensor. The RTK-Ras-Erk signaling pathway regulates a myriad of cellular and developmental processes and is the most frequently mutated signaling pathway in human cancer. Using Drosophila as a model system, we are studying how RTK signaling directs specific gene responses in the nucleus. We have found that the HMG-box protein Capicua (Cic) is an important mediator of such responses. Cic acts antagonistically to RTK signaling in a relatively simple switch: in the absence of signaling, Cic represses RTK-induced targets, whereas RTK activation causes Cic downregulation and derepression of its targets. Importantly, Cic is similarly regulated by RTK signaling in mammals and has been implicated in human neurodegeneration and in various forms of cancer, where it behaves as a tumor suppressor. Recently, we have found that Cic employs a new mode of DNA binding that distinguishes it from other HMG-box factors and explains its mutation patterns in cancer
Transcriptional regulation in Drosophila embryonic patterning. Precise spatio-temporal control of gene expression is at the heart of animal development. We are interested in the mechanisms and factors that mediate this control during Drosophila development. In particular, we have studied several tiers of transcriptional regulation for the establishment and patterning of the Drosophila anteroposterior and dorsoventral embryonic axes, including a mechanism in which binding of Cic to distinct DNA sites controls the simultaneous spatial subdivision of both axes.
Function and specificity of transcriptional corepressors. We also have a long-term interest in eukaryotic transcriptional repression and corepressors. Corepressors usually serve as a link between DNA binding transcriptional repressors and the actual downstream mechanisms leading to transcriptional repression, such as chromatin compaction. However, what dictates the specificity of corepressors –their involvement in one particular regulatory context or another– is not well understood in most cases. We are addressing this question by studying the activities of two conserved corepressors, Groucho/TLE and Atrophin.
Lab people

Gerardo Jiménez
Principal investigator
Gerardo Jiménez is an ICREA Research Professor. He graduated in Biology from the University of Barcelona and obtained his PhD in 1993 for work carried out in the laboratories of Tariq Enver and Mel Greaves at the Institute of Cancer Research in London.
He then worked as a postdoctoral fellow in the laboratory of David Ish-Horowicz at Cancer Research UK, in Oxford and in London. Since then, his research has centered on the molecular mechanisms and pathways that regulate gene expression during animal development. He is head of the Gene expression and signaling laboratory since 2002.
Past students
Laura Rodríguez
Clàudia Lagares
Marta Forés
Aikaterini Papagianni
María José Andreu
Leiore Ajuría
Emma Cervera Tena
Sofía Gómez
Selected publications
Rodríguez-Muñoz L, Lagares C, González-Crespo S, Castel P, Veraksa A, Jiménez G (2022)
Noncanonical function of Capicua as a growth termination signal in Drosophila oogenesis.
Proc Natl Acad Sci USA 119, e2123467119. doi: 10.1073/pnas.2123467119
Cuevas-Navarro A, Rodriguez-Muñoz L, Grego-Bessa J, Cheng A, Rauen KA, Urisman A, McCormick F, Jiménez G, Castel P (2022)
Cross-species analysis of LZTR1 loss-of-function mutants demonstrates dependency to RIT1 orthologs.
eLife 11, e76495. doi: 10.7554/eLife.76495
Papagianni A, Forés M, Shao W, He S, Koenecke N, Andreu MJ, Samper N, Paroush Z, González-Crespo S, Zeitlinger J, Jiménez G (2018)
Capicua controls Toll/IL-1 signaling targets independently of RTK regulation.
Proc Natl Acad Sci USA 115, 1807-1812. doi: 10.1073/pnas.1713930115
Simón-Carrasco L, Graña O, Salmón M, Jacob HKC, Gutierrez A, Jiménez G, Drosten M, Barbacid M (2017)
Inactivation of Capicua in adult mice causes T-cell lymphoblastic lymphoma.
Genes Dev. 31, 1456-1468. doi: 10.1101/gad.300244.117
Forés M, Simón-Carrasco L, Ajuria L, Samper N, González-Crespo S, Drosten M, Barbacid M, Jiménez G (2017)
A new mode of DNA binding distinguishes Capicua from other HMG-box factors and explains its mutation patterns in cancer.
PLoS Genetics 13, e1006622. doi: 10.1371/journal.pgen.1006622
Yang L, Sayantanee P, Trieu KG, Dent LG, Froldi F, Forés M, Webster K, Siegfried KR, Kondo S, Harvey K, Cheng LY, Jiménez G, Shvartsman SY, Veraksa A (2016)
Minibrain and Wings apart control organ growth and tissue patterning through downregulation of Capicua.
Proc Natl Acad Sci USA 113, 10583-10588. doi: 10.1073/pnas.1609417113
Samee MAH, Lim B, Samper N, Lu H, Rushlow CA, Jiménez G, Shvartsman SY, Sinha S (2015)
A systematic ensemble approach to thermodynamic modeling of gene expression from sequence data.
Cell Systems 1, 396-407. doi: 10.1016/j.cels.2015.12.002
Jin Y, Ha N, Forés M, Xiang J, Gläβer C, Maldera J, Jiménez G, Edgar BA (2015)
EGFR/Ras signaling controls Drosophila intestinal stem cell proliferation via Capicua-regulated genes.
PLoS Genetics 11, e1005634. doi: 10.1371/journal.pgen.1005634
Forés M, Ajuria L, Samper N, Astigarraga S, Nieva C, Grossman R, González-Crespo S, Paroush Z, Jiménez G (2015)
Origins of context-dependent gene repression by Capicua.
PLoS Genetics 11, e1004902. doi: 10.1371/journal.pgen.1004902
Lim B, Samper N, Lu H, Rushlow C, Jiménez* G, Shvartsman* SY (2013)
Kinetics of gene derepression by ERK signaling.
Proc Natl Acad Sci USA 110, 10330-10335. doi: 10.1073/pnas.1303635110
Andreu MJ, González-Pérez E, Ajuria L, Samper N, González-Crespo S, Campuzano S, Jiménez G (2012)
Mirror represses pipe expression in follicle cells to initiate dorsoventral axis formation in Drosophila.
Development 139, 1110-1114. doi: 10.1242/dev.076562
Jiménez G, Shvartsman SY, Paroush Z (2012)
The Capicua repressor – a general sensor of RTK signaling in development and disease.
J Cell Sci 125, 1383-1391. doi: 10.1242/jcs.092965
Ajuria L, Nieva C, Winkler C, Kuo D, Samper N, Andreu MJ, Helman A, González-Crespo S, Paroush Z, Courey AJ, Jiménez, G (2011)
Capicua DNA-binding sites are general response elements for RTK signaling in Drosophila.
Development 138, 915-924. doi: 10.1242/dev.057729
Helman A, Cinnamon E, Mezuman S, Hayouka Z, Von Ohlen T, Orian A, Jiménez G, Paroush Z. (2011)
Phosphorylation of Groucho mediates RTK feedback inhibition and prolonged pathway target gene expression.
Curr Biol 21, 1102-1110. doi: 10.1016/j.cub.2011.05.043
Kim Y, Paroush Z, Nairz K, Hafen E, Jiménez* G, Shvartsman* SY (2011)
Substrate- dependent control of MAPK phosphorylation in vivo.
Mol Syst Biol 7, 467. doi: 10.1038/msb.2010.121
Astigarraga S, Grossman R, Díaz-Delfín J, Caelles C, Paroush Z, Jiménez G (2007)
A MAPK docking site is critical for downregulation of Capicua by Torso and EGFR RTK signaling.
EMBO J 26, 668-677. doi: 10.1038/sj.emboj.7601532
Morán E, Jiménez G (2006)
The Tailless nuclear receptor acts as a dedicated repressor in the early Drosophila embryo.
Mol Cell Biol 26, 3446-3454. doi: 10.1128/MCB.26.9.3446-3454.2006
All publications
Rodríguez-Muñoz L, Lagares C, González-Crespo S, Castel P, Veraksa A, Jiménez G (2022)
Noncanonical function of Capicua as a growth termination signal in Drosophila oogenesis.
Proc Natl Acad Sci USA 119, e2123467119. doi: 10.1073/pnas.2123467119
Cuevas-Navarro A, Rodriguez-Muñoz L, Grego-Bessa J, Cheng A, Rauen KA, Urisman A, McCormick F, Jiménez G, Castel P (2022)
Cross-species analysis of LZTR1 loss-of-function mutants demonstrates dependency to RIT1 orthologs.
eLife 11, e76495. doi: 10.7554/eLife.76495
Papagianni A, Forés M, Shao W, He S, Koenecke N, Andreu MJ, Samper N, Paroush Z, González-Crespo S, Zeitlinger J, Jiménez G (2018)
Capicua controls Toll/IL-1 signaling targets independently of RTK regulation.
Proc Natl Acad Sci USA 115, 1807-1812. doi: 10.1073/pnas.1713930115
Simón-Carrasco L, Jiménez* G, Barbacid M, Drosten* M (2018)
The Capicua tumor suppressor: a gatekeeper of Ras signaling in development and cancer.
Cell Cycle 17, 702-711. doi: 10.1080/15384101.2018.1450029
Simón-Carrasco L, Graña O, Salmón M, Jacob HKC, Gutierrez A, Jiménez G, Drosten M, Barbacid M (2017)
Inactivation of Capicua in adult mice causes T-cell lymphoblastic lymphoma.
Genes Dev 31, 1456-1468. doi: 10.1101/gad.300244.117
Forés M, Simón-Carrasco L, Ajuria L, Samper N, González-Crespo S, Drosten M, Barbacid M, Jiménez G (2017)
A new mode of DNA binding distinguishes Capicua from other HMG-box factors and explains its mutation patterns in cancer.
PLoS Genetics 13, e1006622. doi: 10.1371/journal.pgen.1006622
Jiménez G (editor) (2017)
ERK Signaling: Methods and Protocols.
Methods Mol Biol 1487, Springer, New York. doi: 10.1007/978-1-4939-6424-6
Forés M., Papagianni A, Rodríguez-Muñoz L, Jiménez G (2017)
Using CRISPR-Cas9 to study ERK signaling in Drosophila.
Methods Mol Biol 1487, 353-365. doi: 10.1007/978-1-4939-6424-6_26
Yang L, Sayantanee P, Trieu KG, Dent LG, Froldi F, Forés M, Webster K, Siegfried KR, Kondo S, Harvey K, Cheng LY, Jiménez G, Shvartsman SY, Veraksa A (2016)
Minibrain and Wings apart control organ growth and tissue patterning through downregulation of Capicua.
Proc Natl Acad Sci USA 113, 10583-10588. doi: 10.1073/pnas.1609417113
Samee MAH, Lim B, Samper N, Lu H, Rushlow CA, Jiménez G, Shvartsman SY, Sinha S (2015)
A systematic ensemble approach to thermodynamic modeling of gene expression from sequence data.
Cell Systems 1, 396-407. doi: 10.1016/j.cels.2015.12.002
Jin Y, Ha N, Forés M, Xiang J, Gläβer C, Maldera J, Jiménez G, Edgar BA (2015)
EGFR/Ras signaling controls Drosophila intestinal stem cell proliferation via Capicua-regulated genes.
PLoS Genetics 11, e1005634. doi: 10.1371/journal.pgen.1005634
Forés M, Ajuria L, Samper N, Astigarraga S, Nieva C, Grossman R, González-Crespo S, Paroush Z, Jiménez G (2015)
Origins of context-dependent gene repression by Capicua.
PLoS Genetics 11, e1004902. doi: 10.1371/journal.pgen.1004902
Lim B, Samper N, Lu H, Rushlow C, Jiménez* G, Shvartsman* SY (2013)
Kinetics of gene derepression by ERK signaling.
Proc Natl Acad Sci USA 110, 10330-10335. doi: 10.1073/pnas.1303635110
Andreu MJ, Ajuria L, Samper N, González-Pérez E, Campuzano S, González-Crespo S, Jiménez G (2012)
EGFR-dependent downregulation of Capicua and the establishment of Drosophila dorsoventral polarity.
Fly 6, 234-239. doi: 10.4161/fly.21160
Andreu MJ, González-Pérez E, Ajuria L, Samper N, González-Crespo S, Campuzano S, Jiménez G (2012)
Mirror represses pipe expression in follicle cells to initiate dorsoventral axis formation in Drosophila.
Development 139, 1110-1114. doi: 10.1242/dev.076562
Jiménez G, Shvartsman SY, Paroush Z (2012)
The Capicua repressor – a general sensor of RTK signaling in development and disease.
J Cell Sci 125, 1383-1391. doi: 10.1242/jcs.092965
Helman A, Lim B, Andreu MJ, Kim Y, Shestkin T, Lu H, Jiménez G, Shvartsman SY, Paroush Z (2012)
RTK signaling modulates the Dorsal gradient.
Development 139, 3032-3039. doi: 10.1242/dev.075812
Helman A, Cinnamon E, Mezuman S, Hayouka Z, Von Ohlen T, Orian A, Jiménez G, Paroush Z. (2011)
Phosphorylation of Groucho mediates RTK feedback inhibition and prolonged pathway target gene expression.
Curr Biol 21, 1102-1110. doi: 10.1016/j.cub.2011.05.043
Kim Y, Andreu MJ, Lim B, Chung K, Terayama M, Jiménez G, Berg CA, Lu H, Shvartsman SY (2011)
Gene regulation by MAPK substrate competition.
Dev Cell 20, 880-887. doi: 10.1016/j.devcel.2011.05.009
Ajuria L, Nieva C, Winkler C, Kuo D, Samper N, Andreu MJ, Helman A, González-Crespo S, Paroush Z, Courey AJ, Jiménez, G (2011)
Capicua DNA-binding sites are general response elements for RTK signaling in Drosophila.
Development 138, 915-924. doi: 10.1242/dev.057729
Kim Y, Paroush Z, Nairz K, Hafen E, Jiménez* G, Shvartsman* SY (2011)
Substrate- dependent control of MAPK phosphorylation in vivo.
Mol Syst Biol 7, 467. doi: 10.1038/msb.2010.121
Kim Y, Coppey M, Grossman R, Ajuria L, Jiménez G, Paroush Z and Shvartsman SY (2010).
MAPK substrate competition integrates patterning signals in the Drosophila embryo.
Curr Biol 20, 446-451. doi: 10.1016/2Fj.cub.2010.01.019
Cinnamon E, Helman A, Ben-Haroush Schyr R, Orian A, Jiménez G, Paroush Z (2008)
Multiple RTK pathways downregulate Groucho-mediated repression in Drosophila embryogenesis.
Development 135, 829-837. doi: 10.1242/dev.015206
Astigarraga S, Grossman R, Díaz-Delfín J, Caelles C, Paroush Z, Jiménez G (2007)
A MAPK docking site is critical for downregulation of Capicua by Torso and EGFR RTK signaling.
EMBO J 26, 668-677. doi: 10.1038/sj.emboj.7601532
Morán E, Jiménez G (2006)
The Tailless nuclear receptor acts as a dedicated repressor in the early Drosophila embryo.
Mol Cell Biol 26, 3446-3454. doi: 10.1128/MCB.26.9.3446-3454.2006
Kidd T, Abu-Shumays R, Katzen A, Sisson JC, Jiménez G, Pinchin S, Sullivan W, Ish-Horowicz D (2005)
The ε-subunit of ATP synthase is required for normal spindle orientation during the Drosophila embryonic divisions.
Genetics 170, 697-708. doi: 10.1534/genetics.104.037648
Cinnamon E, Gur-Wahnon D, Helman A, St Johnston D, Jiménez G, Paroush Z (2004)
Capicua integrates input from two maternal systems in Drosophila terminal patterning.
EMBO J 23, 4571-4582. doi: 10.1038%2Fsj.emboj.7600457
Roch F, Jiménez G, Casanova J (2002)
EGFR signalling inhibits Capicua-dependent repression during specification of Drosophila wing veins.
Development 129, 993-1002. doi: 10.1242/dev.129.4.993
Jiménez G, González-Reyes A, Casanova J (2002)
Cell surface proteins Nasrat and Polehole stabilize the Torso-like extracellular determinant in Drosophila oogenesis.
Genes Dev 16, 913-918. doi: 10.1101%2Fgad.223902
Jiménez G, Guichet A, Ephrussi A, Casanova J (2000)
Relief of gene repression by Torso RTK signaling: role of capicua in Drosophila terminal and dorsoventral patterning.
Genes Dev 14, 224-231. PMID: 10652276
Eberhard D, Jiménez G, Heavy B, Busslinger M (2000)
Transcriptional repression by Pax5 (BSAP) through interaction with corepressors of the Groucho family.
EMBO J 19, 2292-2303. doi: 10.1093/emboj/19.10.2292
Goldstein RE, Jiménez G, Cook O, Gur D, Paroush Z (1999)
Huckebein repressor activity in Drosophila terminal patterning is mediated by Groucho.
Development 126, 3747-3755. doi: 10.1242/dev.126.17.3747
Jiménez G, Verrijzer CP, Ish-Horowicz D (1999)
A conserved motif in Goosecoid mediates Groucho-dependent repression in Drosophila embryos.
Mol Cell Biol 19, 2080-2087. doi: 10.1128/mcb.19.3.2080
Jiménez G, Paroush Z, Ish-Horowicz D (1997)
Groucho acts as a corepressor for a subset of negative regulators, including Hairy and Engrailed.
Genes Dev 11, 3072-3082. doi: 10.1101%2Fgad.11.22.3072
Jiménez G, Ish-Horowicz D (1997)
A chimeric Enhancer-of-split transcriptional activator drives neural development and achaete-scute expression.
Mol Cell Biol 17, 4355-4362. doi: 10.1128/mcb.17.8.4355
Jiménez G, Pinchin SM, Ish-Horowicz D (1996)
In vivo interactions of the Drosophila Hairy and Runt transcriptional repressors with target promoters.
EMBO J 15, 7088-7098. doi: 10.1002/j.1460-2075.1996.tb01100.x
Enver T, Li Q, Gale KB, Hu M, May GE, Karlinsey JE, Jiménez G, Papayannopoulou T, Costantini F (1994)
Analysis of the developmental and transcriptional potentiation functions of 5’HS2 of the murine β-globin locus control region in transgenic mice.
Dev Biol 165, 574-584. doi: 10.1006/dbio.1994.1277
Jiménez G, Ford AM, Enver T, Boronat A (1993)
Multiple changes in chromatin structure precede the transcriptional activation of the human growth hormone locus in placental cells.
Mol Cell Endocrinol 96, 53-60. doi: 10.1016/0303-7207(93)90094-Z
Jiménez G, Griffiths S, Ford AM, Greaves MF, Enver T (1992)
Activation of the β-globin locus control region precedes commitment to the erythroid lineage.
Proc Natl Acad Sci USA 89, 10618-10622. doi: 10.1073/pnas.89.22.10618
Jiménez G, Gale KB, Enver T (1992)
The mouse β-globin locus control region: hypersensitive sites 3 and 4.
Nucleic Acids Res 20, 5797-5803. doi: 10.1093/nar/20.21.5797
Jiménez G, Ford AM, Boronat A (1992)
SphI RFLP at the human growth hormone gene cluster
Nucleic Acids Res 20, 1169. doi: 10.1093/nar/20.5.1169
Project funding
2021-2024
Transcriptional repression in Drosophila development
Funded by the Spanish Government (Ref. PID2020-119248GB-I00)
Role: PI
Proyecto PID2020-119248GB-I00 financiado por:

2018-2020
Transcriptional interpretation of RTK signaling
Funded by the Spanish Government (Ref. BFU2017-87244-P)
Role: PI
Proyecto BFU2017-87244-P financiado por:

2018-2020
Chromatin and gene expression
Funded by the Catalan Government (Ref. 2017 SGR 475)
Role: Co-PI
2015-2017
Transcriptional regulation by RTK signaling
Funded by the Spanish Government (Ref. BFU2014-52863-P)
Role: PI
2013-2016
Molecular analysis of Capicua, a novel tumor suppressor involved in RTK signaling and transcriptional repression
Funded by the Marató de TV3 (Ref. 20131730)
Role: Co-PI and Coordinator
2003-2014
Four consecutive projects funded by the Spanish Government
Role: PI
Vacancies/Jobs
We welcome enquiries from prospective undergraduate students, PhD students and postdoctoral researchers. Please contact us by e-mail (gjcbmc@ibmb.csic.es).
(November 2022)
A research student position is available in our group to work on gene expression and genome organization during animal development.We are seeking a highly motivated candidate who is currently enrolled or has recently completed a Master degree in the Life Sciences. Ideally, candidates should hold a bachelor degree in Biology, Biochemistry or a related area, with an average score above 2.5/4.0 (8.25/10).
We offer a one-year contract equivalent to the FPU (MICINN) or FI (AGAUR) fellowships, with possibility of extension.
Inquiries and applications may be addressed to Dr. Gerardo Jiménez (gjcbmc@ibmb.csic.es).
Lab corner
There are currently no items in this folder.