Lab presentation
We (@Nek967) are interested in understanding how cell division is controlled, and more specifically how before and during mitosis a number of signaling modules reorganize cellular elements with the objective of building the spindle and correctly segregating the duplicated chromosomes into two daughter cells. This is evidently quite an important process -think cell death, disease and mayhem in general if things go wrong with it. The microtubule cytoskeleton, associated proteins including molecular motors and the centrosomes (in animal cells) are central to it and thus we pay special attention to them
We are focused on studying the NIMA-family kinases Nek9, Nek6 and Nek7. We have shown that during G2/M Nek9 is activated at centrosomes and then it binds and activates the highly similar Nek6 and Nek7. Once active the three kinases can modify different substrates, controlling their localization and/or function. We use genetically modified cells and animals to investigate the importance of this for both centrosome and spindle function. We are also interested in understanding how other members of the NIMA family such as Nek8 signal in the context of the primary cilium.
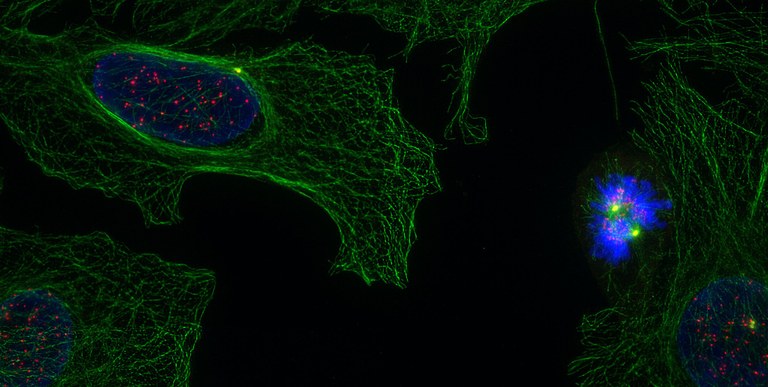
Human U2OS cells; note the cell in prometaphase, building the mitotic spindle through the reorganization of its microtubule cytoskeleton (tubulin in green, centrosomes in yellow, kinetochores in red, DNA in blue; image: Núria Gallisà).
Projects
What we do (if you are not a biological scientist): we study how cells are wired, that is, how different systems similar to wires and switches control the elements that make up a cell by transmitting signals and changing the behavior, location or amount of these elements (we like to say that we study signal transduction).
More specifically we are interested in understanding how a particular type of signal (protein phosphorylation) is involved in controlling how cells divide. (Applications? you may want to know that aberrant control of cell division is at the very heart of, for example, cancer)
Background
Mitosis is a highly controlled biological process. A variety of signals regulate multiple elements both in time and space to ensure that chromosomes are segregated in two equal groups into daughter cells. Cells prepare for mitosis in G2, and in response to CDK1 activation undergo different changes, including the reorganization of the microtubule apparatus to build the mitotic spindle. Failure to properly control spindle formation and chromosome segregation may result in aneuploidy, one of the most frequent features of cancer cells.
We study how protein phosphorylation, alongside other signals such as ubiquitination or the Ran(GTP) gradient, control progression through mitosis and the formation of the mitotic spindle (see for example Walczak and Heald 2008) through the modification of different protein types, for example proteins that control the growth or disposition of the microtubules. These proteins often accumulate at the centrosome, the main microtubule organizing center of animal cells. The centrosome is a cytoplasmic non-membranous organelle that contains two microtubule-based cylinders (the centrioles) surrounded by a structured cloud of protein (the pericentriolar material or PCM). When present (e.g. in animal cells) the centrosome is the major place of novel microtubule nucleation (see Teixidó-Travesa et al 2012) and organization, playing a key role during spindle formation. Centrosomes also have a major role in establishing cell polarity and enabling asymmetrical division (e.g. in stem cells) as well as during cilia formation (Bornens 2012).
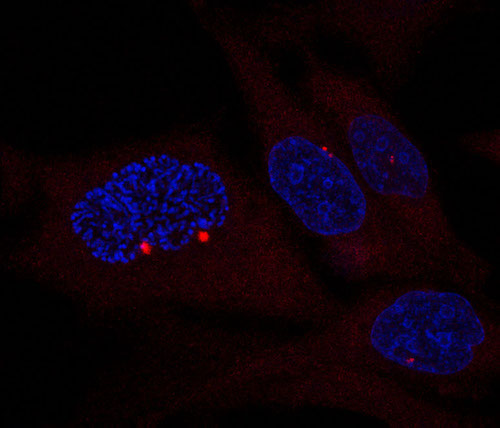
Centrosomes (visualized by γ-tubulin staining in red; DNA in blue) in a prophase HeLa cell (left) as compared to interphase cells (right). Note size and separation of duplicated centrosomes in prophase. Image: Sara Sdelci.
Of the different families of enzymes controlling spindle formation and mitotic progression, one of the least studied is the NIMA family of protein kinases. The NIMA family comprises 11 members (Nek1-11) in mammals (see O’Connell et al. 2003). Of these, Nek2 is involved in the regulation of the centrosome cycle in G2, while Nek9 and the highly similar Nek6 and Nek7 are after our work known to form a signaling module that is crucial for the normal formation of the mitotic spindle (see below, and Fry et al. 2012 for a recent review on cell cycle regulation by Neks).
Nek9 is a 120 kDa NIMA kinase that contains an autoinhibitory RCC1 domain followed by a C-terminal tail that functions as an oligomerization domain and binds among other proteins to the related Nek6 and Nek7.
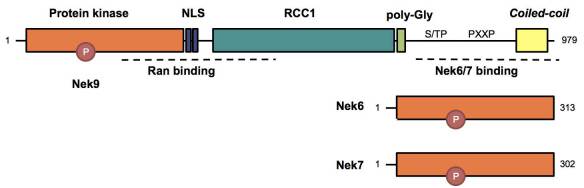
We have shown that a small fraction of Nek9 is activated during mitosis at the centrosomes by a mechanism involving phosphorylation by two of the major mitotic regulators, CDK1 and Plk1 (Bertran et al 2011). Once active, Nek9 autophosphorylates and is able to bind and activate Nek6/7.We have shown that a small fraction of Nek9 is activated during mitosis at the centrosomes by a mechanism involving phosphorylation by two of the major mitotic regulators, CDK1 and Plk1 (Bertran et al 2011). Once active, Nek9 autophosphorylates and is able to bind and activate Nek6/7.
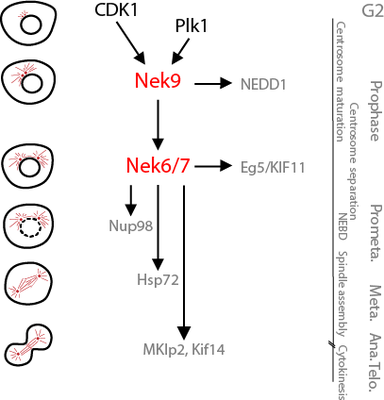
Nek9, Nek6 and Nek7 have been shown by us and others to be necessary for normal spindle formation. We presently study the molecular basis of this and have found that, downstream of Nek9, Nek6/7 control centrosome separation during early mitosis through the regulation of the centrosomal accumulation of Eg5, a molecular motor of the kinesin family that is crucial for bipolar spindle formation (Rapley et al. 2008; Bertran et al 2011; for a review on Eg5 see Ferenz et al. 2012).Nek9 and Nek6/7 do this by regulating Eg5 ability to interact with TPX2, a multifunctional MAP and by controlling TPX2 subcellular localization (Eibes et al. 2017).
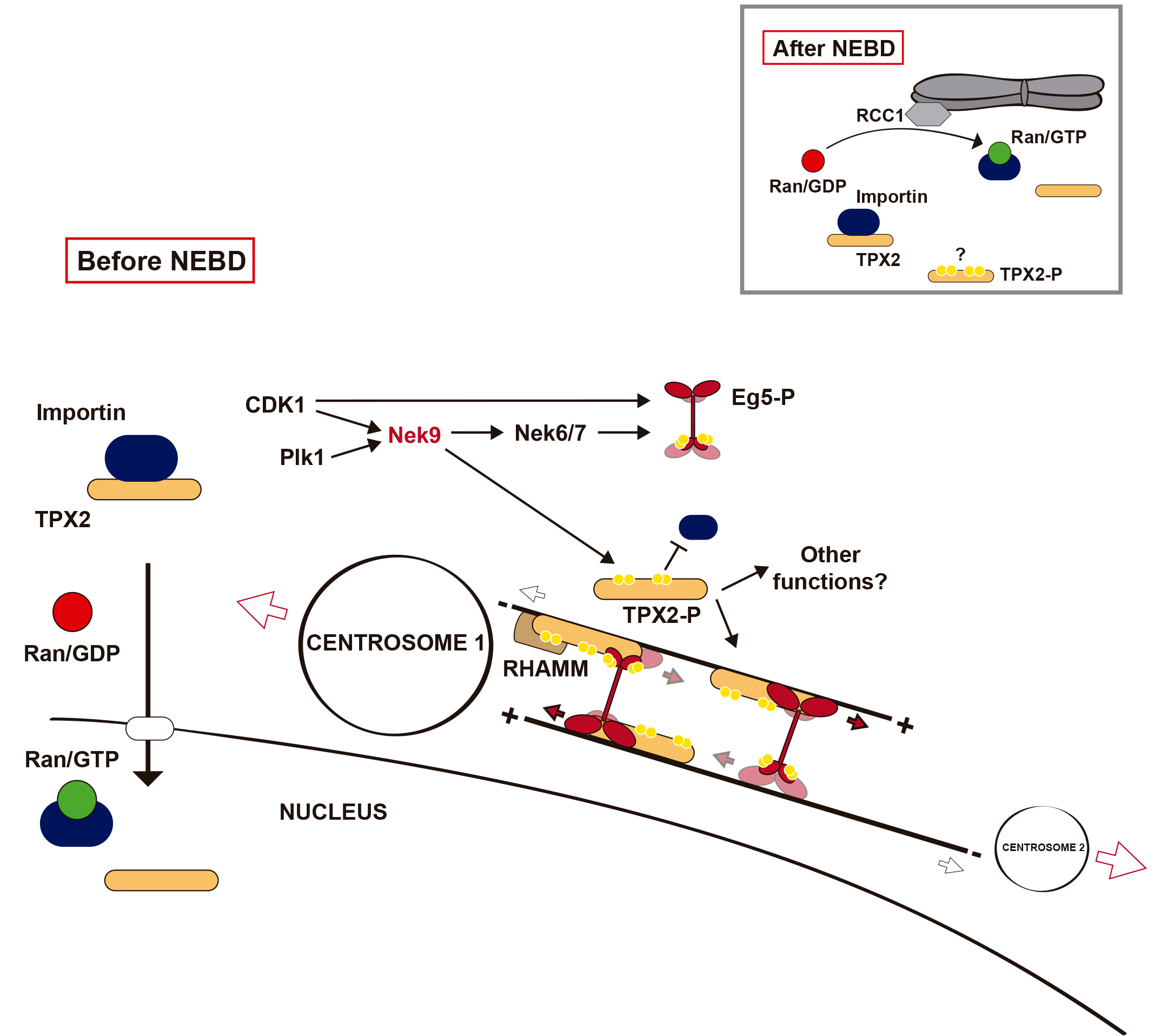 In collaboration with the Vernos group at the CRG, we have also shown that simultaneously Nek9 controls the ability of the centrosomes to nucleate novel microtubules by regulating the centrosomal accumulation of Nedd1/GCP-WD, an adaptor for the microtubule nuceating complex γ-TuRC (Sdelci et al. 2012; for an overview on nucleation see Teixidó-Travesa et al 2012).
In collaboration with the Vernos group at the CRG, we have also shown that simultaneously Nek9 controls the ability of the centrosomes to nucleate novel microtubules by regulating the centrosomal accumulation of Nedd1/GCP-WD, an adaptor for the microtubule nuceating complex γ-TuRC (Sdelci et al. 2012; for an overview on nucleation see Teixidó-Travesa et al 2012).
Research Lines
- Known and novel functions of the Nek9/Nek6/7 module:
We would like to understand the functions of the Nek9/Nek6/7 module in different cell types and physiopathological contexts. For this we use different interfering techniques in both cells and organisms and seek to enumerate the substrates of the kinases using high throughput proteomic techniques combined with more classical approaches. The final objective is to understand how phosphorylation by Nek9 or Nek6/7 affect substrate behavior and how this is used by the cell to perform different tasks. An example of this would be the phosphorylation of the molecular motor Eg5 by Nek6/7, that through the interaction with a number of proteins (including the MAP TPX2, also phosphorylated by Nek9) accumulates around centrosomes making posible the separation of these organelles at the beginning of mitosis -and thus the rapid and accurate segregation of chromosomes to daughter cells.
- Nek9, Nek6 and Nek7 in pathological states:
We investigate the possible implication of Nek9, Nek6 and Nek7 in cellular transformation and cancer onset as well as in other human pathologies, and assess their interest as clinical targets.
- Phosphorylation and signaling in G2 and M:
We are generally interested in understanding how phosphorylation modulates the execution of G2/M, including the organization of the mitotic spindle, as well as how this is integrated with the control of the centrosomal cycle.
Lab people

Joan Roig
Principal investigator
Joan Roig is the principal investigator of the Cell Cycle and Signaling group at the Institute of Molecular Biology of Barcelona (IBMB). Dr. Roig graduated in Biology in 1993 from the Universitat Autònoma de Barcelona (UAB), where he developed a long-standing interest in protein kinases and the study of intracellular signaling through protein phosphorylation.
After obtaining his PhD he moved to the United States where he worked as a postdoctoral fellow at the University of California at Riverside (1998-2000) and the Massachusetts General Hospital / Harvard University in Boston (2000-2005), under the direction of Drs. Jolinda Traugh and Joseph Avruch respectively.
Supported by different fellowships, including one from the Leukemia and Lymphoma Society, Dr. Roig continued to study different protein kinases, identifying Nek9, a novel member of the NIMA family, that was subsequently found to activate the related Nek6 and Nek7.
Through the Ramon y Cajal and the I3 programs Dr. Roig established an independent research group in Barcelona to study these and other related kinases and their importance in the regulation of the G2 and Mphases of the cell cycle as well as the centrosomal cycle, initially at the Institut de Recerca Biomèdica (IRB Barcelona) and after being awarded a CSIC research position in 2016, at the IBMB.

Laura Regué Barrufet

Lídia Montes Ruiz

Sanaz Panjo
Past students
Celia Lerma
Meritxell Serra
Paula Martínez
Antonio Lorenzo Martínez Wambre
Núria Campos
Marta Redondo
Bruna Frielink Immich
Susana Eibes
Priscilla Ferreira Papa
Fernanda Luisa Basei
Cristina Vila
Sara Sdelci
Neus Teixidó
M. Teresa Bertran
Laura Regué
Paula Sánchez Fernández de Landa
Núria Gallisà Suñé
Salut Colell Muñoz
Lorena Fernández de Larrea
Lídia Montes Ruiz
Saber Haroufi
Selected publications
Fry, A., Bayliss, R. and Roig, J. (2017). Mitotic regulation by NEK kinase networks. Frontiers in Cell and Developmental Biology 5: 102. doi: 10.3389/fcell.2017.00102
Eibes, S., Gallisà-Suñé, N., Rosas-Salvans, M., Martínez- Delgado, P., Vernos, I. and Roig,J. (2017) Nek9 phosphorylation defines a new role for TPX2 in Eg5- dependent centrosome separation before nuclear envelope breakdown. Current Biology 28 pii: S0960-9822(17)31528-2. doi: 10.1016/j.cub.2017.11.046
Sdelci, S., Schutz, M., Pinyol, R., Bertran, M.T., Regué, L., Caelles, C. Vernos, I. and Roig, J. (2012). Nek9 phosphorylation of NEDD1/GCP-WD contributes to Plk1 control of γ-tubulin recruitment to the mitotic centrosome. Current Biology 22, 1516-1523. doi: 10.1016/j.cub.2012.06.027
Bertran, M.T., Sdelci, S., Regué, L., Avruch, J., Caelles, C. and Roig, J. (2011). Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5. The EMBO Journal 30, 263-2647. doi: 10.1038/emboj.2011.179
Regué, L., Sdelci, S., Bertran, M.T., Caelles, C., Reverter, D. and Roig, J. (2011). DYNLL/LC8 controls signal transduction through the Nek9/Nek6 signaling module by regulating Nek6 binding to Nek9. Journal of Biological Chemistry 286, 18118-18129. doi: 10.1074/jbc.M110.209080
All publications
Pashley SL, Papageorgiou S, O’Regan L, Barone G, Robinson SW, Lucken K, Straatman KR, Roig J, Fry AM (2024). The mesenchymal morphology of cells expressing the EML4-ALK V3 oncogene is dependent on phosphorylation of Eg5 by NEK7. J Biol Chem 107144. doi: 10.1016/j.jbc.2024.107144
Gallisà-Suñé N, Sànchez-Fernàndez-de-Landa P, Zimmermann F, Serna M, Regué L, Paz J, Llorca O, Lüders J, Roig J (2023). BICD2 phosphorylation regulates dynein function and centrosome separation in G2 and M. Nat Commun 14:1–20. doi: 10.1038/s41467-023-38116-1
Kamranvar SA, Gupta DK, Wasberg A, Liu L, Roig J, Johansson S. (2022). Integrin-Mediated Adhesion Promotes Centrosome Separation in Early Mitosis. Cells 11:1360. doi:10.3390/cells11081360
Gallisà-Suñé N, Sànchez-Fernàndez-de-Landa P, Zimmermann F, Serna M, Paz J, Regué L, Llorca O, Luders J, Roig J. (2021). CDK1 and PLK1 cooperate to regulate BICD2, dynein activation and recruitment to the nucleus as well as centrosome separation in G2/M. bioRxiv. doi: 10.1101/2021.11.04.467245
Eisa NH, Jilani Y, Kainth K, Redd P, Lu S, Bougrine O, Abdul Sater H, Patwardhan CA, Shull A, Shi H, Liu K, ElSherbiny NM, Eissa LA, El-Shishtawy MM, Horuzsko A, Bollag R, Maihle N, Roig J, Korkaya H, Cowell JK, Chadli A. (2019). The co-chaperone UNC45A is essential for the expression of mitotic kinase NEK7 and tumorigenesis. J Biol Chem. doi:10.1074/jbc.RA118.006597
Freixo F, Martinez Delgado P, Manso Y, Sánchez-Huertas C, Lacasa C, Soriano E, Roig J & Lüders J (2018) NEK7 regulates dendrite morphogenesis in neurons via Eg5-dependent microtubule stabilization. Nat Commun 9: 2330. Pubmed (featured in the IRB page)
Eibes, S., Gallisà-Suñé, N., Rosas-Salvans, M., Martínez- Delgado, P., Vernos, I. and Roig,J. (2017) Nek9 phosphorylation defines a new role for TPX2 in Eg5- dependent centrosome separation before nuclear envelope breakdown. Curr. Biol. Dec 13. pii: S0960-9822(17)31528-2. Pubmed
Cota, R.R., Teixidó-Travesa, N., Ezquerra, A., Eibes, S., Lacasa, C., Roig, J. and Lüders, J. (2017) MZT1 regulates microtubule nucleation by linking γTuRC assembly to adapter-mediated targeting and activation. J. Cell. Sci. 130:406-419. Pubmed
Haq, T., Richards, M.W., Burgess, S.G., Gallego, P., Yeoh, S., O’Regan, L., Reverter, D., Roig, J., Fry, A.M. and Bayliss, R. (2105) Mechanistic basis of Nek7 activation through Nek9 binding and induced dimerization. Nat. Commun. 2, 6:8771. PubMed
Januschke, J., Reina, J., Llamazares, S., Bertran, T., Rossi, F., Roig, J. and Gonzalez, C. (2013) Centrobin controls mother-daughter centriole asymmetry in Drosophila neuroblasts. Nat. Cell. Biol., 15, 241-8. PubMed
Gallego, P., Velazquez-Campoy, A., Regue, L., Roig, J. and Reverter, D. (2013) Structural analysis of the regulation of the DYNLL/LC8 binding to Nek9 by phosphorylation. J. Biol. Chem. 288, 12283-12294. PubMed (featured in the ALBA Synchrotron and IRB pages)
Sdelci, S., Schutz, M., Pinyol, R., Bertran, M.T., Regué, L., Caelles, C. Vernos, I. and Roig, J. (2012). Nek9 phosphorylation of NEDD1/GCP-WD contributes to Plk1 control of γ-tubulin recruitment to the mitotic centrosome. Curr. Biol. 22, 1516-1523. PubMed (Artículo del mes, Spanish Society of Biochemistry and Molecular Biology, SEBBM)
Bertran, M.T., Sdelci, S., Regué, L., Avruch, J., Caelles, C. and Roig, J. (2011). Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5. EMBO J. 30, 263-2647. PubMed (Featured in Cell Cycle, see below)
Regué, L., Sdelci, S., Bertran, M.T., Caelles, C., Reverter, D. and Roig, J. (2011). DYNLL/LC8 controls signal transduction through the Nek9/Nek6 signaling module by regulating Nek6 binding to Nek9. J. Biol. Chem. 286, 18118-18129. PubMed
Teixidó-Travesa, N., Villén, J., Lacasa, C., Bertran, M.T., Archinti, M., Gygi, S., Caelles, C., Roig, J. and Jens Lüders (2010). The γTuRC revisited: a comparative analysis of interphase and mitotic human γTuRC re-defines the set of core components and identifies the novel subunit GCP8. Mol. Biol. Cell. 21, 3963-3972. PubMed
Rapley, J., Nicolàs, M., Groen, A., Regué, L., Bertran, M.T., Caelles, C., Avruch, J. and Roig, J. (2008). The NIMA-family kinase Nek6 phosphorylates the kinesin Eg5 at a novel site necessary for mitotic spindle formation. J. Cell Sci. 121, 3912-21. PubMed
Comments, Reviews and Book Chapters
- Fry, A., Bayliss, R. and Roig, J. Mitotic regulation by NEK kinase networks. Front. Cell Dev. Biol., 01 December 2017 | https://doi.org/10.3389/fcell.2017.00102. Link
- Eibes, S. and Roig, J. (2016) MCRS1: Not only ran. Cell Cycle. 15, 2693-4. Pubmed
- Teixidó-Travesa, N., Roig, J. and Lüders, J. (2012) The where, when and how of microtubule nucleation – one ring to rule them all. J Cell Sci 125: 4445-4456. PubMed
- Sdelci, S., Bertran, M.T. and Roig, J. (2011) Nek9, Nek6, Nek7 and the separation of centrosomes. Cell Cycle. 10, 3816-7. PubMed
- Roig, J. (2010). Nek6. UCSD-Nature Molecule Pages. doi:10.1038/mp.a003029.01. Link
- Roig, J. (2010). Nek7. UCSD-Nature Molecule Pages. doi:10.1038/mp.a003428.01. Link
- Roig, J. (2010). Nek9. UCSD-Nature Molecule Pages. doi:10.1038/mp.a003631.01. Link
Project funding
Active projects
El eje señalizador PLK1/NEK9/NEK6/7 en G2 y mitosis temprana (PID2021-127045NB-
Plan Nacional de I+D, Ministerio de Ciencia e Innovación, Spain, 2022-2024.
Principal Investigator: Joan Roig.
Proyecto PID2021-127045NB-

Past projects (last 10 years)
Las NIMA quinasas Nek9, Nek6 y Nek7. Estudio de sus funciones en celulas, tejidos y el organism (PGC2018-096307-B-I00).
Plan Nacional de I+D, Ministerio de Ciencia, Innovación y Universidades, Spain. 2019-21.
Principal Investigator: Joan Roig.
Proyecto PGC2018-096307-B-I00 financiado por:

Bases Moleculars de Processos Fisiopatològics Fonamentals (2017 SGR 1089).
Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR), Generalitat de Catalunya, Spain. 2018.
Coordinador: Travis Stracker
La quinasa de la familia NIMA Nek9. Funciones, implicacion en la aparicion de aneuploidia y posible uso como una nueva diana de drogas antimitoticas (BFU2014-58422-P).
Plan Nacional de I+D, Ministerio de Economía y Competitividad, Spain. 2015-2017.
Principal Investigator: Joan Roig
High Throughput screening for Nek9 inhibitors I-III.
Obra Social “La Caixa”. 2014-2016.
Principal Investigator: Joan Roig
The NIMA-family kinase Nek9 as a putative anti-mitotic drug target.
Bayer HealthCare. 2014.
Principal Investigator: Joan Roig
Regulación y funciones de las proteína quinasas Nek9, Nek6 and Nek7 (BFU2011-25855).
Plan Nacional de I+D+I, Ministerio de Ciencia e Innovación, Spain. 2012-2014.
Principal Investigator: Joan Roig
El módulo de señalización Nercc1/Nek6/7; regulación y funciones (BFU2008-03441/BMC).
Plan Nacional de I+D+I, Ministerio de Ciencia e Innovación, Spain. 2009-2011.
Principal Investigator: Joan Roig
Vacancies/Jobs
If you are interested in joining the lab as postdoc, PhD or master student please send us your CV and cover letter: Joan Roig (joan.roig@ibmb.csic.es).
Lab corner
PAST STUDENTS
- Lídia Montes Ruiz, Master Student.
- Saber Haroufi, Master Student.
- Salut Colell Muñoz, Master Student.
- Lorena Fernández de Larrea, Master Student.
- Núria Gallisà, PhD student.
- Paula Sánchez Fernández de Landa, Master Student.
- Celia Lerma, Master student.
- Meritxell Serra, TFG student.
- Paula Martínez, PhD student.
PhD Univ. of Barcelona, 2018 “Identification of novel Nek9 substrates and functions through the use of genetically engineered mice. New roles in the control of the centrosome cycle”. - Antonio Lorenzo Martínez Wambre, Master student.
- Núria Campos, Lab Manager.
- Marta Redondo, Master Student.
- Bruna Frielink Immich, PhD student in Guido lenz and Dr. Jenifer Saffi’s labs (Sandwich Doctorate fellowship, CNPq, Brazil)
Now finishing her PhD a postdoctoral researcher in Porto Alegre, Brazil. - Susana Eibes, PhD student.
PhD Univ. of Barcelona, 2016 “Functional study of the NIMA Protein Kinases Nek9, Nek6 and Nek7 at the onset of mitosis”.
Present location: Danish Cancer Society Research Center, Copenhagen, Denmark. - Priscilla Ferreira Papa, PhD student in Jörg Kobarg’s lab (Research Internship Abroad, FAPESP, São Paulo Research Foundation, Brazil).
- Fernanda Luisa Basei, PhD student in Jörg Kobarg’s lab (Research Internship Abroad, FAPESP, São Paulo Research Foundation, Brazil).
- Cristina Vila, lab manager.
Present location: Esteve, Barcelona. - Sara Sdelci, PhD student (FI-DGR fellowship).
PhD Univ. of Barcelona, 2012 ” Role of the kinases Nek6, Nek7 and Nak9 in the regulation of the centrosome cycle”.
Present location: group leader, CRG Barcelona (http://www.crg.eu/en/programmes-groups/sdelci-lab) - Neus Teixidó, postdoctoral fellow.
Present location: Thomson Reuters, Barcelona. - M. Teresa Bertran, PhD student (FPU fellowship).
PhD Univ. of Barcelona, 2012 “Study of the phosphorylation and activation of the protein kinase Nek9 during mitosis”.
Present location: London Research Intitute, London, UK. - Laura Regué, PhD student.
PhD Univ. of Barcelona, 2011 ” Molecular basis of the regulation of the activity of the Nercc1/Nek9 protein kinase”.
Present location: MGH/HMS, Harvard University, Boston, USA.
NEWS
JOAN ROIG HAS BEEN INTERVIEWED BY EL ESPAÑOL NEWSPAPER TO TALK ABOUT THE PROTEIN NEK8 AND THE PATHOLOGICAL CONSEQUENCES OF ITS MUTATION
JOAN ROIG APPEARED IN TELEMADRID’S MORNING MAGAZINE “BUENOS DÍAS MADRID” TO TALK ABOUT THE NIMA PROTEIN KINASE NEK8
AND SARA GOT HER OWN GROUP!!
NEW COLLABORATIVE PAPER WITH THE LÜDERS LAB.
We (and our mice) were happy to collaborate in the last article from the IRB Microtubule Organization Lab, describing the important roles that Nek7 has for neuron morphology. Interestingly, Nek7 regulates dendrite growth and branching through the control of the kinesin Eg5/KIF11, suggesting that postmitotic cells recycle the Nek6/7/Eg5 signaling module that we previously described for roles other than spindle formation.
Looking forward to continue working with Jens Lüders and his group on this fascinating topic! (Specially because we like his Batman T-shirt).
See also here.
NEW PAPER: MORE ON SEPARATION AND A NEW MODE OF REGULATION FOR TPX2.
Our latest paper on the Neks and centrosome separation is online (link). And the journal cover has a rhino in it! (a female eastern black rhino).
We describe the role of Eg5 phosphorylation by the Neks and in doing so a new mode of regulation for the multifunctional TPX2 -by, of course, phosphorylation. Another great collaboration with the group of Isabelle Vernos at the CRG. Now to figure out whether phosphorylation may be regulating other SAFs similarly, by interfering with the action of the importin/Ran system…
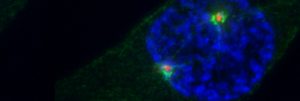
A prophase (centrosomes, R; Eg5, G; DNA, B)
You can read about it also here.
NEW REVIEW
Feel like you need to know more about the mitotic Neks? read the review we wrote with Andrew Fry and Richard Bayliss in Frontiers in Cell and Developmental Biology (link).
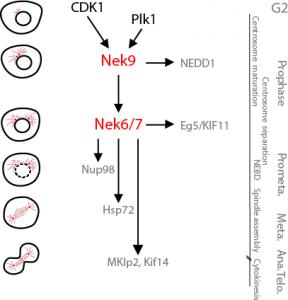
Nek9, Nek6 and Nek7 roles during mitosis.
WE MOVED FROM THE IRB TO THE IBMB, THE MOLECULAR BIOLOGY INSTITUTE OF BARCELONA FROM THE CSIC. AND TO DO THAT WE DIDN’T EVEN NEED TO CHANGE BUILDING!
You can find us now in the S1C51 lab.
NO, WE ARE NOT DANCING WITH THE IRB FOR CANCER, ALZHEIMER’S AND DIABETES RESEARCH. WE ARE SHY. BUT WE LIKE THE VIDEO!
WE CONTRIBUTED TO CAYETANO GONZÁLEZ GROUP WORK ON THE MOLECULAR BASIS OF ASYMETRIC CELL DIVISION, THAT WAS PUBLISHED IN NATURE CELL BIOLOGY
OUR COLLABORATION WITH THE GROUP OF DAVID REVERTER AT THE UAB RESULTED IN ONE OF THE FIRST PROTEIN STRUCTURES OBTAINED FROM DATA COLLECTED AT THE ALBA SYNCHROTON
OUR WORK IN COLLABORATION WITH ISABELLE VERNOS GROUP AT THE CRG WAS PUBLISHED IN CURRENT BIOLOGY - AND SARA WAS ON TV
https://www.irbbarcelona.org/en/news/catalan-researchers-identify-a-key-component-of-cell-division
National media run several stories about it (see for example this) and Sara became a TV star:
https://www.irbbarcelona.org/en/news/irb-barcelonas-breakthrough-in-cell-division-on-tv3