Lab presentation
Historically, we have been investigating the role of the JNK signalling cascade in multiple processes during the Drosophila and zebrafish development. In particular, we established histoblasts in Drosophila as a model for the analysis of cell cycle control, cell invasiveness, planar cell polarity and the mechanical control of cell replacement. In these studies, we pioneered the application of FRET sensors technology into high throughput RNAi screenings.
In the last few years, we have shifted to explore more global problems, trying to understand how functional integration between system levels (from genes and proteins to higher physiological processes) works for building an organism. Nowadays we only have little cues on how to comprehend systems-level interactions during development. To reach a holistic physical and biological understanding of morphogenesis is not an easy task. Our goal is to shed light into emergent new properties consequence of the interactions in between different levels applying genetic, cellular and modelling tools.
Our laboratory studies the processes controlling morphogenesis employing Drosophila as a model system, with a special emphasis on how collective cell migration is regulated, and the biomechanics of coordinated morphogenetic events. The current interest of the lab is centred in two main issues: the mechanisms ruling the coordination of the simultaneous morphogenesis of different tissues to build a single functional structure (nerves, muscles and epidermis in the rebuilding of the fly abdomen during metamorphosis); and the mechanical control of the development and morphogenesis of the embryonic Central Nervous System (CNS). These analyses demand quantitative parametric studies of cellular and physiological functions not achievable without a combination of imaging, experimental genetics, cell behavior analysis, nanotechnology and mathematical modelling.
The laboratory includes 3 scientists and currently runs two grants funded by the Spanish and the Catalan Governments. Over the years we have kept a high impact publishing profile (Science, Genes & Development, Developmental Cell, Current Biology, Development, PLOS Biology, PLOS Genetics, Cell Reports and EMBO Journal). In addition, our group has acted as coordinator of an EU FP6 project, participated in CONSOLIDER grants and was part of the organizing committees of the 8th European Zebrafish Conference and the 23rd European Drosophila Research Conference. The group is a member of the Mechanobiology Spanish Network.
Projects
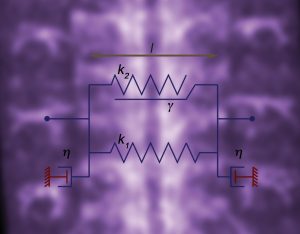
Mechanical properties and forces in the CNS of Drosophila
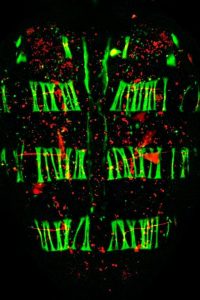
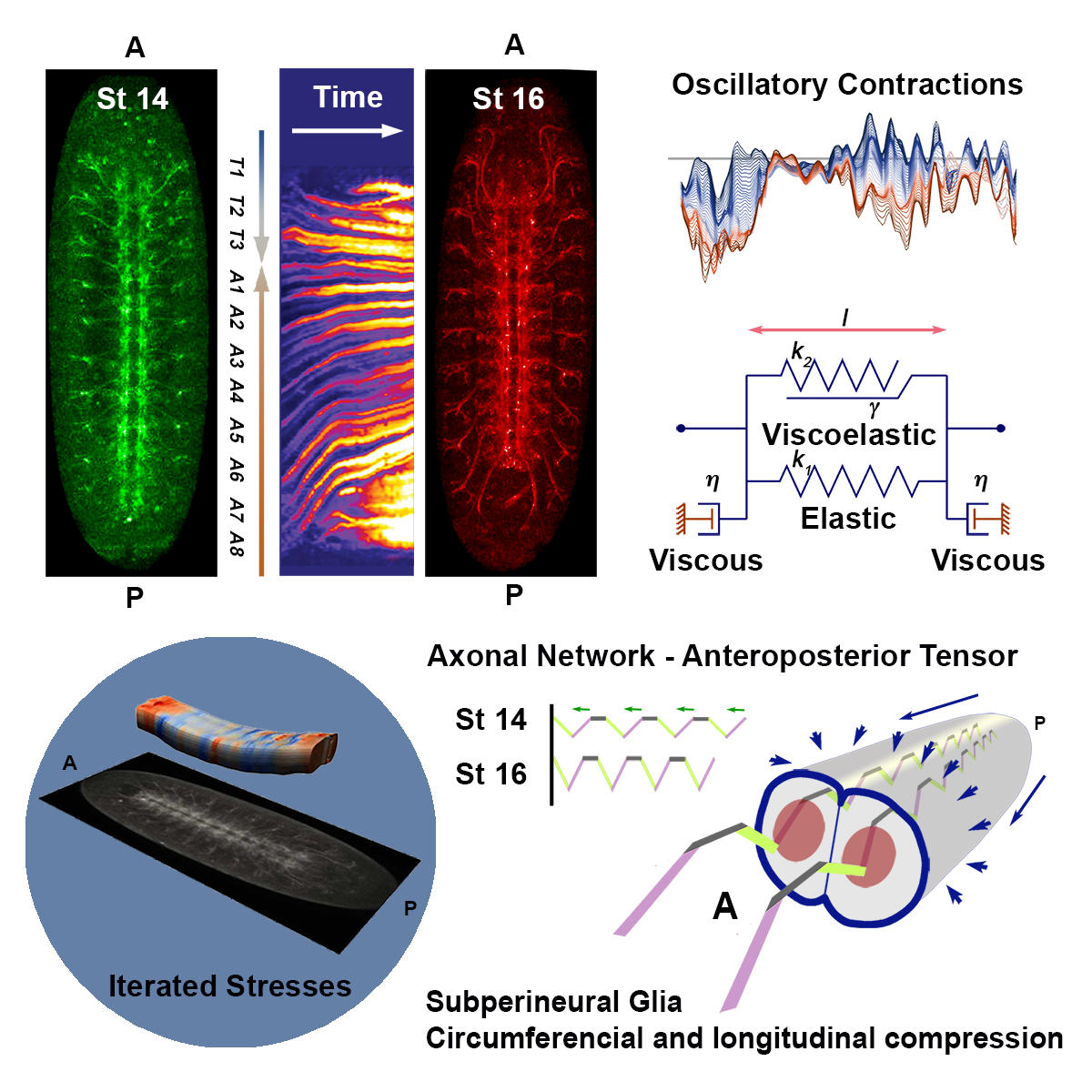
The insect nervous system is built stepwise, making use of cell driven precise and identifiable processes. Macroscopically, the Drosophila CNS undergoes an early elongation associated to germ band extension and a dramatic late condensation that further progresses in the larvae. During condensation, the VNC neuromeres gradually condense into engrossed units separated by sulci resulting in a reduction of the VNC length up to 25%. VNC condensation is dependent on neuronal apoptosis, glial scaffold integrity and ECM deposition by hemocytes. Simultaneously to condensation, the Blood Brain Barrier assembly is initiated. During this process, the outermost layer of glial cells (perineural and sub-perineural glia), link through septate junctions, gradually insulating the CNS from the hemolymph. Drosophila neurons excitability is very sensitive to the extracellular concentrations of sodium, potassium, and calcium ions and the BBB is critical for their functionality. We have evaluated in detail the mechanics of VNC condensation and it’s worth mentioning that three articles focusing on the analyses on VNC morphogenesis are currently in press or submitted:
Condensation of the Drosophila Nerve Cord is Oscillatory and depends on Coordinated Mechanical Interactions. Karkali K, Tiwari P, Singh A, Tlili S, Jorba I, Navajas D, Muñoz JJ, Saunders TE, Martin-Blanco Developmental Cell (in Press)
JNK signaling in pioneer neurons directs the architectural organization of the CNS of the Drosophila embryo. Karkali K, Saunders TE, Panayotou G, Martín-Blanco E. (Submitted)
Puckered in pioneer neurons coordinates the motor activity of the Drosophila embryo. Karkali K, Vernon SW, Baines RA, Panayotou G and Martín-Blanco E. (Submitted)
Despite their obvious morphological differences, the Drosophila and human developing brains share several similarities. From an anatomical point of view, the VNC, could be considered as the functional counterpart of the spinal cord and VNC condensation could be compared, to some extent, to the process of rhombomere formation or, more distantly, to cortex gyrification. All three processes relay on biomechanical inputs, tissue growth and cell-cell rearrangements. Gyrification increases the cortex surface area, promoting cognitive capacity. Similarly, VNC condensation brings closer the different VNC neural modules promoting circuitry optimization and motor coordination.
In summary, the Drosophila embryonic CNS development shares key characteristics with the development of the human brain. It relies on conserved cellular processes, follows equivalent organization parameters and has functionally related modular blocks. One of our aims is to exploit VNC condensation as a model for studying the genetic bases modulating the structural mechanics of neural tissue morphogenesis. In this regard, CNS geometry, size and shape, structural stability and functional optimization depend, both in Drosophila and humans, on biomechanical actions. Taking this into consideration, analyzing the structural roles of neurodevelopmental disorder (NDD) related genes in this model could provide insights into the cellular and mechanical basis of human brain congenital malformations.
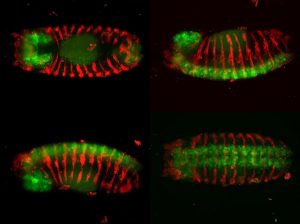
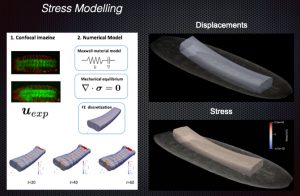
Coordination of neural, muscular and epithelial morphogenesis during the morphogenesis of the adult abdomen
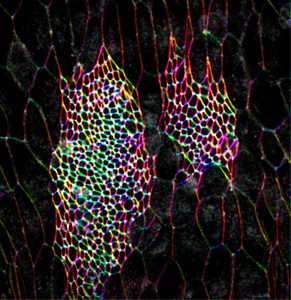
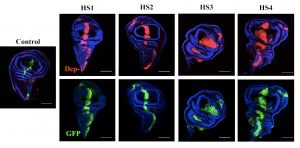
Epithelial tissues participate in organ formation and the establishment of the body shape by organizing themselves into sheets, tubules or vesicles. They carry three essential functions: protect the organism for the external environment, provide mechanical and structural support, and maintain and compartmentalize individual environments. In holometabolous insects such as Drosophila, during metamorphosis, the larval external epithelium is completely replaced and this process relies on maintaining a balance between adult precursors (histoblasts) proliferation and growth and the elimination of larval epithelial cells (LECs) so that the integrity of the epithelium is ensured. Histoblasts spreading and the apical constriction of LECs are non-autonomous processes and their coordination results from a mutual exchange of signals.
We have previously explored in depth this model and, in particular, its mechanical aspects. In these analyses we identified and characterized strains and stresses patterns and uncovered the properties of the mechanical crosstalk between histoblasts and LECs. We also appreciated that the consequences of this mechanical feedback were not limited to the replacement of the latter by the former but expanded to further aspects of the morphogenesis of the adult abdomen. These include amongst others the 3D folding of the epithelial tissue and the specification and patterning of the sensory organs that decorate the epidermis. The patterning of newborn muscles and adult heart, built from myoblasts precursors, at the expense of the larval muscles also depend, in great part, on the remodeling of the epithelial landscape, as it does the pruning and rewiring of segmental and intersegmental nerves. Our aim in this project is to analyze these events, characterize their dynamics and study their biomechanics.
Regeneration versus Tumorogenesis
During healing and regeneration cells communicate and migrate to close wounds employing the same mechanisms they use during morphogenesis. Drosophila imaginal discs are a very good model for studies of regeneration. Directed genetic ablation leads disc cells to change fate and fulfill complete regeneration. Multiple studies, including our own, have clarified in great detail those molecular mechanisms and cellular processes involved in early healing and in the late regenerative responses. Yet, key aspects on the control of regeneration faithfulness are unknown. Tissue regeneration has to be precise and we do not know how precision is achieved.
Effective and conserved tissue-repair processes can become spontaneously misregulated and a close association between chronic tissue damage, failing to heal and regenerate, inflammation and cancer has been repeatedly observed. Tumors have been described as “wounds that won’t heal,” pointing to many similarities between cancerous tumor growth and wound healing 9. There is mounting evidence that many tumors are propagated by cancer stem cells with a virtually infinite capacity for self-renewal. Other tumors arise from transformation of normal stem cells into cancer stem cells. If this holds true, tissue stem cells should be closely guarded against insults and might be particularly vulnerable to deregulated immunity. Thus, the tumor-propagating potential of cancer stem cells may relate to their ability to hijack natural self-renewal programs as those stimulated during tissue regeneration. Regeneration implies the tightly regulated control of different features, including proliferation, polarity and patterning. If these, one or several, become uncontrolled they may cause tumors. Indeed, a great number of cases of wound-related cancers have been reported. Still, no detailed analysis of the association between repair and regeneration and the origin of tumors has been reported.
Gene conservation allows for the use of model organisms in the study of genetic pathologies. This becomes especially evident when wound healing or tumorigenesis in vertebrates and Drosophila are compared. Tumor formation can be triggered in Drosophila in many ways, including oncoprotein overexpression (RasV12). RasV12 in imaginal discs produces overgrowths and, with simultaneous loss of apico-basal proteins like Scribbled (Scrib), generates multi-layered amorphous masses with high growth rate and metastatic properties.
We hypothesize that by manipulating regenerating cells we could study the factors that might trigger tumors. Indeed, in Drosophila, we, amongst others, have found a strong parallelism between the initial steps of regeneration and tumor formation. We have also identified a subset of evolutionarily conserved genes whose expression is essential for healing. Our preliminary analyses (see below) show that experimentally inducing multiple regeneration events, in response to sequential apoptotic death induction, leads to the uncontrolled proliferation of discs cells. They lose apicobasal polarity, the basal membrane gets disrupted and the overproliferating epithelial tissue is infiltrated by migratory hemocytes. In this scenario, in this project, we aim to focus in analyzing how the imaginal disc regenerating tissue could become transformed into an abnormal hyperproliferative mass of cells with potential invasive capabilities.
Lab people

Enrique Martín-Blanco
I received my Ph.D. in Biological Sciences in 1986. Afterwards I moved to UCSF with Tom Kornberg and characterized the structure of the complex of Engrailed homeodomain with DNA, the first protein/DNA complex resolved in eukaryotes. Returning to Spain in 1991, I joined Antonio García-Bellido’s lab at the CBMSO, where I focused on the analysis of left-right asymmetry control. After receiving an offer from Alfonso Martinez-Arias, I went to Cambridge in 1993. There, I studied how different morphogenetic processes are regulated, uncovering fundamental principles in cell communication. These studies set the basis of my career as independent Researcher (Cientifico Titular (2001) and Investigador Cientifico (2009) in the IBMB (CSIC). I have also spent extended specialization periods at the MPI for Biophysical Chemistry (2004 – Tom Jovin), Institut Curie (2008 -Yohanns Bellaiche), Mechanobiology Institute of Singapore (2014 – Michael Sheetz), HHMI Janelia (2015 – Eric Betzig) and MPI for Cell and Developmental Genetics (2018 -Eli Knust). At the IBMB I have studied the developmental role of the JNK signalling cascade in multiple processes and revisited histoblasts development as a model for the analysis of cell cycle control, cell invasivity, planar cell polarity and the mechanical control of cell replacement. We also studied collective cell movements in zebrafish. Nowadays, we are applying multidisciplinary approaches to study morphogenesis in Drosophila.
We are very interested on coordinated organogenesis and on the links between architecture and function, with a special emphasis on the Nervous System.

Katerina Karkali

Tanroop Kaur

Luís Merchante

Merna Al Obaidli
Past students
Ariadna Arasanz
Giulia Mencattelli
Alejandro Sebastián Sánchez
Stella Kyriakidou
Selected publications
Top Ten original publications of the Team in the last 10 years
1. Condensation of the Drosophila Nerve Cord is Oscillatory and depends on Coordinated Mechanical Interactions Karkali, K, Tiwari, P, Singh, A, Tlili, S, Jorba, I, Navajas, D, Muñoz, JJ, Saunders, TE and Martin-Blanco, E. Developmental Cell 2022 (in Press) D21-00379R3. doi: 10.1016/j.devcel.2022.03.007
2. Dissection of the Regulatory Elements of the complex expression pattern of Puckered, a dual specificity JNK phosphatase. Karkali, K and Martin-Blanco, E. International Journal of Molecular Sciences 2021; 22(22): 12205. doi: 10.3390/ijms222212205
3. Mechanical coordination is sufficient to promote tissue replacement during metamorphosis in Drosophila. Prat-Rojo C, Pouille PA, Buceta J, Martin-Blanco E. The EMBO Journal 2020; e103594. doi: 10.15252/embj.2019103594
4. The Dachsous/Fat/Four-Jointed Pathway Directs the Uniform Axial Orientation of Epithelial Cells in the Drosophila Abdomen. Mangione F, Martín-Blanco E. Cell Reports 2018; 25(10): 2836-2850.e4. doi: 10.1016/j.celrep.2018.11.036
5. Contractility, differential tension and membrane removal lead zebrafish epiboly biomechanics. Marsal M, Hernández-Vega A, Martin-Blanco E. Cell Cycle 2017;16(14): 1328-1335. doi: 10.1080/15384101.2017.1327489
6. Polarized cortical tension drives zebrafish epiboly movements. Hernández-Vega A, Marsal M, Pouille PA, Tosi S, Colombelli J, Luque T, Navajas D, Pagonabarraga I, Martín-Blanco E. The EMBO Journal 2017; 36(1): 25-41. doi: 10.15252/embj.201694264
7. Identification and functional analysis of healing regulators in Drosophila. Álvarez-Fernández C, Tamirisa S, Prada F, Chernomoretz A, Podhajcer O, Blanco E, Martín-Blanco E. PLoS Genetics 2015; 11(2): e1004965. doi: 10.1371/journal.pgen.1004965
8. Plasticity of the MAPK signaling network in response to mechanical stress. Pereira AM, Tudor C, Pouille PA, Shekhar S, Kanger JS, Subramaniam V, Martín-Blanco E. PLoS One 2014; 9(7): e101963. doi: 10.1371/journal.pone.0101963
9. Integrin-dependent activation of the JNK signaling pathway by mechanical stress. Pereira AM, Tudor C, Kanger JS, Subramaniam V, Martin-Blanco E. PLoS One 2011; 6(12): e26182. doi: 10.1371/journal.pone.0026182
10. Dpp signaling directs cell motility and invasiveness during epithelial morphogenesis. Ninov N, Menezes-Cabral S, Prat-Rojo C, Manjón C, Weiss A, Pyrowolakis G, Affolter M, Martín-Blanco E. Current Biology 2010; 20(6):513-20. doi: 10.1016/j.cub.2010.01.063
Top Five publications in collaboration and/or review articles in the last 10 years
1. ECM degradation in the Drosophila abdominal epidermis initiates tissue growth that ceases with rapid cell cycle exit. Davis JR, Ainslie AP, Williamson JJ, Ferreira A, Torres-Sanchez A, Hoppe A, Mangione F, Smith MB, Martin-Blanco E, Salbreux G, Tapon N. Current Biology 2022; 22: 00107-5. doi: 10.1016/j.cub.2022.01.045
2. Mechanosensing in the Drosophila nervous system. Karkali K, Martin-Blanco E. Seminars in Cell & Developmental Biology 2017; 71: 22-29. doi: 10.1016/j.semcdb.2017.06.014
3. Live imaging in Drosophila: The optical and genetic toolkits. Rebollo E, Karkali K, Mangione F, Martín-Blanco E. Methods 2014; 68(1): 48-59. doi: 10.1016/j.ymeth.2014.04.021
4. Distinct tissue-specific requirements for the zebrafish tbx5 genes during heart, retina and pectoral fin development. Pi-Roig A, Martin-Blanco E, Minguillon C. Open Biology 2014; 4(4):140014. doi: 10.1098/rsob.140014
5. The BTB-zinc finger transcription factor abrupt acts as an epithelial oncogene in Drosophila melanogaster through maintaining a progenitor-like cell state. Turkel N, Sahota VK, Bolden JE, Goulding KR, Doggett K, Willoughby LF, Blanco E, Martin-Blanco E, Corominas M, Ellul J, Aigaki T, Richardson HE, Brumby AM. PLoS Genetics 2013; 9(7): e1003627. doi: 10.1371/journal.pgen.1003627
All publications
1. Karkali, K., Saunders, T.E., Panayotou, G., Martin-Blanco, E. JNK signaling in pioneer neurons organizes ventral nerve cord architecture in Drosophila embryos. Nat Commun 14, 675 (2023). https://doi.org/10.1038/
2. Katerina Karkali; Ignasi Jorba; Daniel Navajas; Enrique Martín-Blanco. Measuring ventral nerve cord stiffness in live flat-dissected Drosophila embryos by atomic force microscopy. STAR Protocols. Volume 3, Issue 4. 2022. doi: 10.1016/j.xpro.2022.101901
3. Condensation of the Drosophila Nerve Cord is Oscillatory and depends on Coordinated Mechanical Interactions. Developmental Cell. 57 – 7, Cell Press, 2022. doi: 10.1016/j.devcel.2022.03.007
4. ECM degradation in the Drosophila abdominal epidermis initiates tissue growth that ceases with rapid cell-cycle exit. Current Biology. 32 – 6, pp. 1285 – 1300. 2022. doi: 10.1016/j.cub.2022.01.045
5. Karkali, Katerina; Martin-Blanco, Enrique. Dissection of the Regulatory Elements of the Complex Expression Pattern of Puckered, a Dual-Specificity JNK Phosphatase. International Journal of Molecular Sciences. 22 – 22, pp. 12205. 2021. doi: 10.3390/ijms222212205
6. María Marsal; Amayra Hernández Vega; Philippe Alexandre Pouille; Enrique Martín Blanco. Rab5ab-Mediated Yolk Cell Membrane Endocytosis Is Essential for Zebrafish Epiboly and Mechanical Equilibrium During Gastrulation. Frontiers in Cell and Developmental Biology. 10.3389/fcell.2021.6, Frontiers, 2021. doi: 10.3389/fcell.2021.697097
7. Mangione, Federica; Martin-Blanco, Enrique. Imaging and Analysis of Tissue Orientation and Growth Dynamics in the Developing Drosophila Epithelia During Pupal Stages. JOVE-. 2020. ISSN 1940-087X. doi: 10.3791/60282
8. Prat-Rojo, Carla; Pouille, Philippe-Alexandre; Buceta, Javier; Martin-Blanco, Enrique. Mechanical coordination is sufficient to promote tissue replacement during metamorphosis in Drosophila. THE EMBO JOURNAL. 39, 2020. ISSN 0261-4189 doi: 10.15252/embj.2019103594
9. Boix-Fabres, Jaume; Karkali, Katerina; Martin-Blanco, Enrique; Rebollo, Elena. Automated Macro Approach to Remove Vitelline Membrane Autofluorescence in Drosophila Embryo 4D Movies. COMPUTER OPTIMIZED MICROSCOPY: METHODS AND PROTOCOLS. 2040, 2019. ISSN 1064-3745, ISBN 978-1-4939-9685-8 doi: 10.1007/978-1-4939-9686-5_9
10. Federica Mangione; Enrique Martin-Blanco. The Dachsous/Fat/Four-Jointed Pathway Directs the Uniform Axial Orientation of Epithelial Cells in the Drosophila Abdomen. CELL REPORTS. 25 – 10, pp. 2836-2850. 12/2018. doi: 10.1016/j.celrep.2018.11.036
11. Katerina Karkali; Enrique Martin-Blanco. Mechanosensing in the Drosophila nervous system. SEMINARS IN CELL & DEVELOPMENTAL BIOLOGY. 71 – SI, pp. 22 – 292017. ISSN 1084-9521. doi: 10.1016/j.semcdb.2017.06.014
12. Amayra Hernández-Vega; María Marsal; Philippe-Alexandre Pouille; Sébastien Tosi; Julien Colombelli; Tomás Luque; Daniel Navajas; Ignacio Pagonabarraga; Enrique Martín-Blanco. Polarized cortical tension drives zebrafish epiboly movements. THE EMBO JOURNAL. pp. e201694264 – e201694264. 2017. doi: 10.15252/embj.201694264
13. Maria Marsal; Ignasi Jorba; Elena Rebollo; Tomas Luque; Daniel Navajas; Enrique Martin-Blanco. AFM and Microrheology in the Zebrafish Embryo Yolk Cell. JOVE. e56224, 2017. doi: 10.3791/56224
14. Maria Marsal; Amayra Hernandez-Vega; Enrique Martin-Blanco. Contractility, differential tension and membrane removal lead zebrafish epiboly biomechanics. CELL CYCLE. 16 – 14, pp. 1328 – 1335. 2017. ISSN 1538-4101. doi: 10.1080/15384101.2017.1327489
15. Enrique Martin-Blanco. Rab5-mediated Yolk Cell Endocytosis modulates Zebrafish Epiboly Biomechanics and Tissue Movements. BioRxiv. 2016. doi: 10.1101/097212
16. Katerina Karkali; George Panayotou; Timothy E Saunders; Enrique Martin-Blanco. The architectural balance of the Ventral Nerve Cord depends on the level of JNK signaling activity. BioRxiv. 2016. doi: 10.1101/092486
17. Álvarez-Fernández C; Tamirisa S; Prada F; Chernomoretz A; Podhajcer O; Blanco E; Martín-Blanco E. Identification and functional analysis of healing regulators in Drosophila. PLOS GENETICS. 11, pp. e100496511. 2015. doi: 10.1371/journal.pgen.1004965
18. Rebollo E; Karkali K; Mangione F; Martín-Blanco E. Live imaging in Drosophila: The optical and genetic toolkits. METHODS. 68, pp. 48 – 59. 06/2014. doi: 10.1016/j.ymeth.2014.04.021
19. Pi-Roig A; Martin-Blanco E; Minguillon C. Distinct tissue-specific requirements for the zebrafish tbx5 genes during heart, retina and pectoral fin development. OPEN BIOLOGY. 4, pp. 140014 – 140014. 2014. doi: 10.1098/rsob.140014
20. Pereira AM; Tudor C; Pouille PA; Shekhar S; Kanger JS; Subramaniam V; Martín-Blanco E. Plasticity of the MAPK signaling network in response to mechanical stress. PLOS ONE. 9, pp. e101963. 2014. doi: 10.1371/journal.pone.0101963
21. Turkel N; Sahota VK; Bolden JE; Goulding KR; Doggett K; Willoughby LF; Blanco E; Martin-Blanco E; Corominas M; Ellul J; Aigaki T; Richardson HE; Brumby AM. The BTB-zinc finger transcription factor abrupt acts as an epithelial oncogene in Drosophila melanogaster through maintaining a progenitor-like cell state. PLOS GENETICS. 9, pp. e1003627. 2013. doi: 10.1371/journal.pgen.1003627
22. Andrea Maria Pereira; Cicerone Tudor; Johannes S. Kanger; Vinod Subramaniam; Enrique Martin-Blanco. Integrin-Dependent Activation of the JNK Signaling Pathway by Mechanical Stress. PLOS ONE. 6 – 12, 2011. doi: 10.1371/journal.pone.0026182
23. Nikolay Ninov; Sofia Menezes-Cabral; Carla Prat-Rojo; Cristina Manjon; Alexander Weiss; George Pyrowolakis; Markus Affolter; Enrique Martin-Blanco. Dpp Signaling Directs Cell Motility and Invasiveness during Epithelial Morphogenesis. CURRENT BIOLOGY. 20 – 6, pp. 513 – 520. doi: 10.1016/j.cub.2010.01.063
24. Nikolay Ninov; Enrique Martin-Blanco. Changing gears in the cell cycle Histoblasts and beyond. FLY. 3 – 4, pp. 286-289. 2009. ISSN 1933-6934. doi: 10.4161/fly.10443
25. Nikolay Ninov; Cristina Manjon; Enrique Martin-Blanco. Dynamic Control of Cell Cycle and Growth Coupling by Ecdysone, EGFR, and PI3K Signaling in Drosophila Histoblasts. PLOS BIOLOGY. 7 – 4, pp. 892 – 903. 2009. ISSN 1544-9173. doi: 10.1371/journal.pbio.1000079
26. A. K. N. Thayil; A. Pereira; M. Mathew; D. Artigas; E. Martin-Blanco; P. Loza-Alvarez. Decrease in laser ablation threshold for epithelial tissue microsurgery in a living Drosophila embryo during dorsal closure. JOURNAL OF MICROSCOPY. 232 – 2, pp. 362 – 368. 2008. ISSN 0022-2720. doi: 10.1111/j.1365-2818.2008.02107.x
27. Chris Bakal; Rune Linding; Flora Llense; Elleard Heffern; Enrique Martin-Blanco; Tony Pawson; Norbert Perrimon. Phosphorylation networks regulating JNK activity in diverse genetic backgrounds. SCIENCE. 322 – 5900, pp. 453 -456. 2008. ISSN 0036-8075. doi: 10.1126/science.1158739
28. Flora Llense; Enrique Martin-Blanco. JNK signaling controls border cell cluster integrity and collective cell migration. CURRENT BIOLOGY. 18 – 7, pp. 538 – 544. 2008. ISSN 0960-9822. doi: 10.1016/j.cub.2008.03.029
29. Nikolay Ninov; Dominic A. Chiarelli; Enrique Martin-Blanco. Extrinsic and intrinsic mechanisms directing epithelial cell sheet replacement during Drosophila metamorphosis. DEVELOPMENT. 134 – 2, pp. 367 – 379. 2007. ISSN 0950-1991. doi: 10.1242/dev.02728
30. Nikolay Ninov; Enrique Martin-Blanco. Live imaging of epidermal morphogenesis during the development of theadult abdominal epidermis of Drosophila. NATURE PROTOCOLS. 2 – 12, pp. 3074 – 3080. 2007. ISSN 1754-2189. doi: 10.1038/nprot.2007.417
31. M Bosch; F Serras; E Martin-Blanco; J Baguna. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. DEVELOPMENTAL BIOLOGY. 280 – 1, pp. 73 – 86. 2005. ISSN 0012-1606. doi: 10.1016/j.ydbio.2005.01.002
32. JC Pastor-Pareja; F Grawe; E Martin-Blanco; A Garcia-Bellido. Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. DEVELOPMENTAL CELL. 7 – 3, pp. 387 – 399. 2004. ISSN 1534-5807. doi: 10.1016/j.devcel.2004.07.022
33. LL Dobens; E Martin-Blanco; A Martinez-Arias; FC Kafatos; LA Raftery. Drosophila puckered regulates Fos/Jun levels during follicle cell morphogenesis. DEVELOPMENT. 128 – 10, pp. 1845 – 1856. 2001. ISSN 0950-1991. doi: 10.1242/dev.128.10.1845
34. E Martin-Blanco; E Knust. Epithelial morphogenesis: Filopodia at work. CURRENT BIOLOGY. 11 – 1, pp. R28 -R31. ISSN 0960-9822. doi: 10.1016/S0960-9822(00)00039-7
35. J Culi; E Martin-Blanco; J Modolell. The EGF receptor and N signalling pathways act antagonistically in Drosophila mesothorax bristle patterning. DEVELOPMENT. 128 – 2, pp. 299 – 308. 2001. ISSN 0950-1991. doi: 10.1242/dev.128.2.299
36. E Martin-Blanco; JC Pastor-Pareja; A Garcia-Bellido. JNK and decapentaplegic signaling control adhesiveness and cytoskeleton dynamics during thorax closure in Drosophila. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA. 97 – 14, pp. 7888 – 7893. 2000. ISSN 0027-8424. doi: 10.1073/pnas.97.14.7888
37. E Martin-Blanco. p38 MAPK signalling cascades: ancient roles and new functions. BIOESSAYS. 22 – 7, pp. 637-645. /2000. ISSN 0265-9247. doi: 10.1002/1521-1878(200007)22:7%3C637::aid-bies6%3E3.0.co;2-e
38. A Baonza; F Roch; E Martin-Blanco. DER signaling restricts the boundaries of the wing field during Drosophila development. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA. 97 – 13, pp. 7331 – 7335. 2000. ISSN 0027-8424. doi: 10.1073/pnas.97.13.7331
39. E Martin-Blanco; F Roch; E Noll; A Baonza; JB Duffy; N Perrimon. A temporal switch in DER signaling controls the specification and differentiation of veins and interveins in the Drosophila wing. DEVELOPMENT. 126 – 24, pp. 5739-5747. 1999. ISSN 0950-1991. doi: 10.1242/dev.126.24.5739
40. E Martin-Blanco; A Martinez-Arias; B Jarvis. Crossreactivity of anti-dual-phosphorylated antibodies with actin. TRENDS IN CELL BIOLOGY. 8 – 10, pp. 419 – 419. 1998. ISSN 0962-8924. doi: 10.1016/s0962-8924(98)01349-x
41. JM Cosgaya; A Aranda; J Cruces; E Martin-Blanco. Neuronal differentiation of PC12 cells induced by engrailed homeodomain is DNA-binding specific and independent of MAP kinases. JOURNAL OF CELL SCIENCE. 111 -Part 16, pp. 2377 – 2384. 1998. ISSN 0021-9533. doi: 10.1242/jcs.111.16.2377
42. F Roch; A Baonza; E Martin-Blanco; A Garcia-Bellido. Genetic interactions and cell behaviour in blistered mutants during proliferation and differentiation of the Drosophila wing. DEVELOPMENT. 125 – 10, pp. 1823 – 1832. 1998. ISSN 0950-1991. doi: 10.1242/dev.125.10.1823
43. E Martin-Blanco; A Gampel; J Ring; K Virdee; N Kirov; AM Tolkovsky; A Martinez-Arias. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. GENES &DEVELOPMENT. 12 – 4, pp. 557 – 570. 1998. ISSN 0890-9369. doi: 10.1101/2Fgad.12.4.557
44. E Martin-Blanco. Regulatory control of signal transduction during morphogenesis in Drosophila. INTERNATIONAL JOURNAL OF DEVELOPMENTAL BIOLOGY. 42 – 3, SI, pp. 363 – 368. 1998. ISSN 0214-6282. PMID: 9654020
45. E MartinBlanco. Regulation of cell differentiation by the Drosophila Jun kinase cascade. CURRENT OPINION IN GENETICS & DEVELOPMENT. 7 – 5, pp. 666 – 671. 1997. ISSN 0959-437X. doi: 10.1016/s0959-437x(97)80015-9
46. E MartinBlanco; A GarciaBellido. Mutations in the rotated abdomen locus affect muscle development and reveal an intrinsic asymmetry in Drosophila. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA. 93 – 12, pp. 6048 – 6052. 1996. ISSN 0027-8424. doi: 10.1073/pnas.93.12.6048
47. HM BOURBON; E MARTINBLANCO; D ROSEN; TB KORNBERG. PHOSPHORYLATION OF THE DROSOPHILA ENGRAILED PROTEIN AT A SITE OUTSIDE ITS HOMEODOMAIN ENHANCES DNA-BINDING. JOURNAL OF BIOLOGICAL CHEMISTRY. 270 – 19, pp. 11130 – 11139. 1995. ISSN 0021-9258. doi: 10.1074/jbc.270.19.11130
48. E MARTINBLANCO; TB KORNBERG. DR-78, A NOVEL DROSOPHILA-MELANOGASTER GENOMIC DNA FRAGMENT HIGHLY HOMOLOGOUS TO THE DNA-BINDING DOMAIN OF THYROID-HORMONE RETINOIC ACID VITAMIN-D RECEPTOR SUBFAMILY. BIOCHIMICA ET BIOPHYSICA ACTA. 1216 – 2, pp. 339 – 341. 1993. ISSN 0006-3002. doi: 10.1016/0167-4781(93)90170-i
49. E MARTINBLANCO; JR VALVERDE; F FLORES; I VERNOS; R MARCO. S1 NUCLEASE-SENSITIVE SITES IN THE BITHORAXOID REGION OF THE DROSOPHILA ULTRABITHORAX GENE. BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS. 194 – 2, pp. 647 – 653. 1993. ISSN 0006-291X. doi: 10.1006/bbrc.1993.1870
50. JM ROJAS; J DOPAZO; E MARTINBLANCO; C LOPEZGALINDEZ; E TABARES. ANALYSIS OF GENETIC-VARIABILITY OF POPULATIONS OF HERPES-SIMPLEX VIRUSES. VIRUS RESEARCH. 28 – 3, pp. 249 – 261. 1993. ISSN 0168-1702. doi: 10.1016/0168-1702(93)90025-i
51. CR KISSINGER; BS LIU; E MARTINBLANCO; TB KORNBERG; CO PABO. CRYSTAL-STRUCTURE OF AN ENGRAILED HOMEODOMAIN-DNA COMPLEX AT 2.8-A RESOLUTION – A FRAMEWORK FOR UNDERSTANDING HOMEODOMAIN-DNA INTERACTIONS. CELL. 63 – 3, pp. 579 – 590. 1990. ISSN 0092-8674. doi: 10.1016/0092-8674(90)90453-l
52. BS LIU; CR KISSINGER; CO PABO; E MARTINBLANCO; TB KORNBERG. CRYSTALLIZATION AND PRELIMINARY-X-RAY DIFFRACTION STUDIES OF THE ENGRAILED HOMEODOMAIN AND OF AN ENGRAILED HOMEODOMAIN DNA COMPLEX. BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS. 171 – 1, pp. 257 – 259. 1990. ISSN 0006-291X. doi: 10.1016/0006-291x(90)91385-6
53. NH PATEL; E MARTINBLANCO; KG COLEMAN; SJ POOLE; MC ELLIS; TB KORNBERG; CS GOODMAN. EXPRESSION OF ENGRAILED PROTEINS IN ARTHROPODS, ANNELIDS, AND CHORDATES. CELL. 58 – 5, pp. 955 – 968. 1989. ISSN 0092-8674. doi: 10.1016/0092-8674(89)90947-1
54. E TABARES; I OLIVARES; G SANTURDE; MJ GARCIA; E MARTIN; ME CARNERO. AFRICAN SWINE FEVER VIRUS-DNA – DELETIONS AND ADDITIONS DURING ADAPTATION TO GROWTH IN MONKEYKIDNEY-CELLS. ARCHIVES OF VIROLOGY. 97 – 3-4, pp. 333 – 346. 1987. ISSN 0304-8608. doi: 10.1007/bf01314431
55. A DOMINGO; M CERVERA; E MARTIN; R MARCO. BIOCHEMICAL-CHARACTERIZATION AND DEVELOPMENTAL BEHAVIOR OF ARTEMIA EMBRYONIC AND NAUPLIAL DEOXYRIBONUCLEASES. BIOCHEMISTRY. 25 – 14, pp. 4125 – 4131. AMER CHEMICAL SOC, 07/1986. ISSN 0006-2960. doi: 10.1021/bi00362a021
56. E TABARES; J MARTINEZ; E MARTIN; JM ESCRIBANO. PROTEINS SPECIFIED BY AFRICAN SWINE FEVER VIRUS .4. GLYCOPROTEINS AND PHOSPHOPROTEINS. ARCHIVES OF VIROLOGY. 77 – 2-4, pp. 167 – 180. 1983. ISSN 0304-8608. doi: 10.1007/bf01309265
Project funding
Resources (last 10 years)
PID2020-116273GB-I00
Ministerio de Ciencia e Innovación.
170.000 Euros
01/09/2021-31/08/2024
PI
Proyecto PID2020-116273GB-I00 financiado por:

2020AEP100
CSIC
25.000 Euros
01/01/2021-31/08/2021
PI
PRX18/00541
Ministerio de Universidades
17.926 Euros
01/09/2018-28/02/2019
PI
BFU2017-82876-P
Ministerio de Ciencia e Innovación.
150.000 Euros
01/01/2018-30/09/2021
PI
Proyecto BFU2017-82876-P financiado por:

2015SGR00845
Grups de Recerca Consolidats
–
01/01/2017-31/12/2021
PI
CMD
Fundación Ramón Areces
127.360 Euros
15/05/2015-14/05/2018
PI
BFU2014-23451
Ministerio de Ciencia y Competividad
135.000 Euros
01/01/2015-31/12/2017
PI
BFU2011-29423
Ministerio de Ciencia y Competividad
386.000 Euros
01/01/2012-31/12/2014
PI
CSD-2007-00008
CONSOLIDER (MECD)
200.734 Euros
01/01/2008-31/12/2013
Co-PI
BFU2008-02769
Ministerio de Ciencia e Innovación
330.000 Euros
01/01/2009-31/12/2011
PI
2009SGR1009
Grups de Recerca Consolidats
22.360 Euros
01/10/2009-30/04/2014
Co-PI
Vacancies/Jobs
We welcome candidates with a strong background in image analysis, modeling, and/or developmental/cell biology, to apply for upcoming calls for international postdoctoral fellowships (EMBO and Marie-Curie), and from the Spanish Government (Juan de la Cierva and Ramon y Cajal),
Please contact us directly to the following email address:
We always look for talented undergraduate and graduate students for internships, TFGs, TFMs, short-term visits and long-term projects.
Lab corner
Project gallery
No albums or photos uploaded yet.
