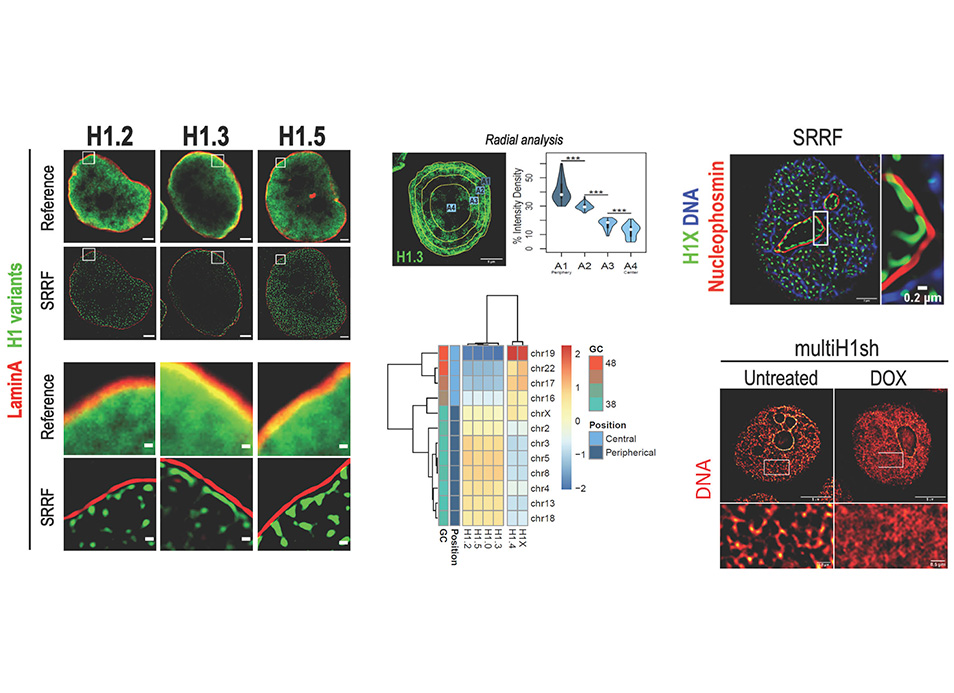
New publication in eLife of the Jordan Lab in collaboration with the IBMB Imaging Platform showing that…
The Chromatin and Gene Regulation Group, led by Dr. Guillermo P. Vicent at IBMB, has recently published an article investigating the influence of the extracellular matrix and the three-dimensional (3D) environment on gene regulation, nuclear structure, and hormonal response in breast cancer cells.
The Chromatin and Gene Regulation Group, led by Dr. Guillermo P. Vicent at IBMB, has recently published an article investigating the influence of the extracellular matrix and the three-dimensional (3D) environment on gene regulation, nuclear structure, and hormonal response in breast cancer cells. The study by Ramírez-Cuéllar et al., featured in the EMBO Journal, introduces new avenues of research into the involvement of the Hippo pathway in estrogen and progesterone-driven cell proliferation in breast cancer. Researchers from the Centre for Genomic Regulation (CRG) and the National Centre for Genomic Analysis (CNAG) in Barcelona, along with the Università di Parma (Italy), contributed to this work.
Abstract
The cancer epigenome has been studied in cells cultured in two-dimensional (2D) monolayers, but recent studies highlight the impact of the extracellular matrix and the three-dimensional (3D) environment on multiple cellular functions. Here, we report the physical, biochemical, and genomic differences between T47D breast cancer cells cultured in 2D and as 3D spheroids. Cells within 3D spheroids exhibit a rounder nucleus with less accessible, more compacted chromatin, as well as altered expression of ~2000 genes, the majority of which become repressed. Hi-C analysis reveals that cells in 3D are enriched for regions belonging to the B compartment, have decreased chromatin-bound CTCF and increased fusion of topologically associating domains (TADs). Upregulation of the Hippo pathway in 3D spheroids results in the activation of the LATS1 kinase, which promotes phosphorylation and displacement of CTCF from DNA, thereby likely causing the observed TAD fusions. 3D cells show higher chromatin binding of progesterone receptor (PR), leading to an increase in the number of hormone-regulated genes. This effect is in part mediated by LATS1 activation, which favors cytoplasmic retention of YAP and CTCF removal.
Synopsis
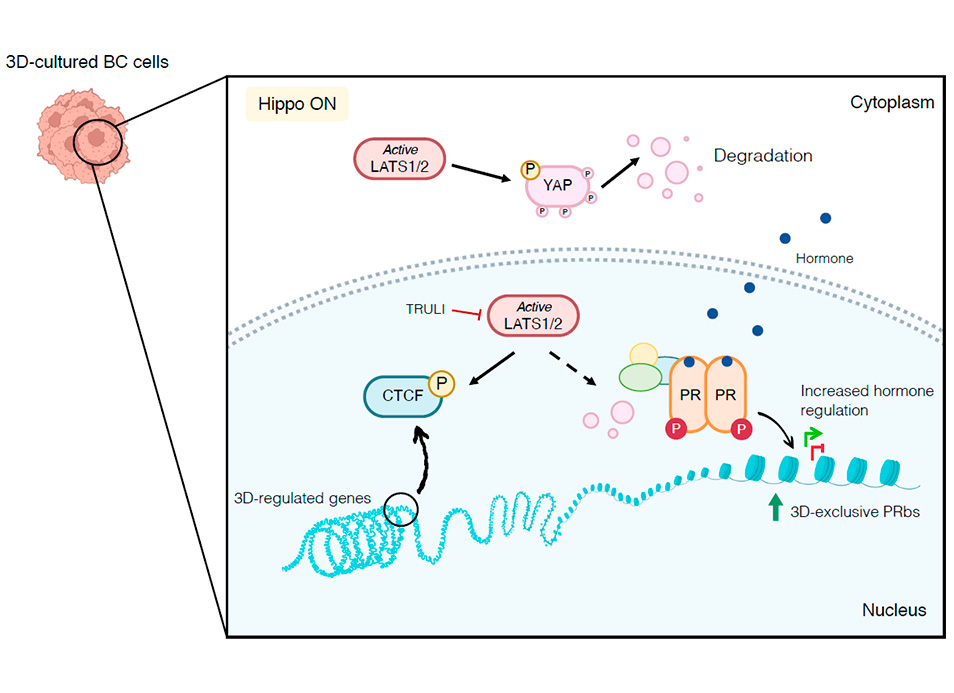
Genomic and epigenomic alterations in cancer cells have so far been mostly studied in 2D monolayer cultures. Here, altered gene expression and chromatin structure linked to LATS1 kinase signaling is found in 3D spheroids grown from breast cancer cells.
•3D-grown cells display less accessible, more compacted chromatin, and altered expression of ~2,000 genes
•3D-grown cells exhibit B-compartment enrichment, reduced chromatin-bound CTCF, and increased topologically associating domain (TAD) fusions.
•Hippo kinase LATS1 activation in 3D promotes CTCF phosphorylation and displacement, likely causing the observed TAD fusions.
•3D cells show elevated progesterone receptor binding, increasing the expression of hormone-regulated genes.
•This increase in the hormone response in 3D cells is partly mediated by LATS1-mediated phosphorylation and cytoplasmic retention of YAP.
Reference:
Julieta Ramírez-Cuéllar, Roberto Ferrari, Rosario T Sanz, Marta Valverde-Santiago, Judith García-García, A Silvina Nacht, David Castillo, Francois Le Dily, Maria Victoria Neguembor, Marco Malatesta, Sarah Bonnin, Marc A Marti-Renom, Miguel Beato and Guillermo P Vicent. LATS1 controls CTCF chromatin occupancy and hormonal response of 3D-grown breast cancer cells. EMBO J (2024). doi: 10.1038/s44318-024-00080-x