Lab presentation
- Mechanisms of neoplasia and malignant progression using cellular and animal models.
- Role of the ubiquitin-proteasome system in the regulation of cell cycle checkpoints and the recognition and repair of DNA damage.
- Design and development of new drugs targeted at regulators of the ubiquitin-proteasome system of interest in cancer and other pathologies.
Projects
Research Lines:
- Study of mechanisms of neoplasia and malignant progression:
Discovery of new genes and pathways relevant in malignancy and in the development of metastasis, useful as markers useful in diagnosis and prognosis and the identification of novel pathways for the development of effective new therapeutic schemes to tackle the problem of metastatic spread of cancer. - Study of the role of the ubiquitin-proteasome system in relevant cellular processes:
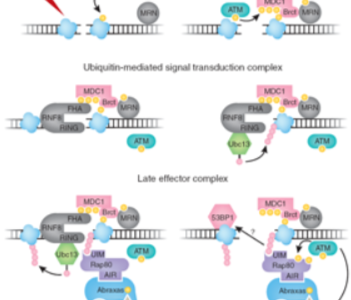
Role of non-canonical ubiquitylation (through K63 of ubiquitin), mediated by the ubiquitin-conjugating enzyme Ubc13-Uev, and role of the ubiquitin ligase RNF8 in checkpoint control and in the establishment of the DNA damage response.
- Drug design and development:
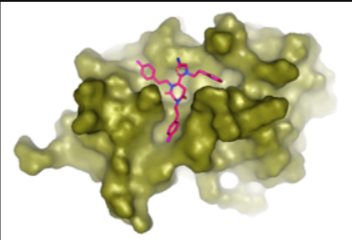
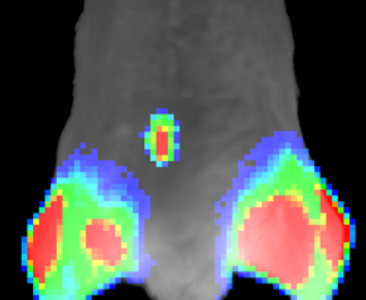
Discovery of novel antagonists of protein-protein interaction with relevance in the regulation of the ubiquitin-proteasome system. After in vitro and in vivo validation in cancer models, our aim is to take compounds with effective anti-tumor activity to preclinical and, eventuall, clinical phases.
Lab people

Timothy Thomson
Principal investigator
T. Thomson is a Board-certified Clinical Immunologist with extensive research experience in tumor immunology, molecular and cellular oncology, regulation of cell signaling by protein ubiquitination and the development and implementation of novel drug discovery schemes.
He discovered the ubiquitin regulators UEV-1 and UEV2, which prompted a definitive shift in paradigm of the ubiquitin-proteasome system from one dedicated to protein destruction to a posttranslational modification with fundamental implications in protein-protein recognition and signaling (Mol Cell Biol 1998; FEBS Lett 1998; Genome Res 2006; J Cell Biochem 2006; Science 2007; Oncogene 2008; PLoS One 2010, Mol Cell 2010).
His lab, in partnership with R. Paciucci, also discovered the adaptor protein PTOV1, which work by R. Pacciucci has placed at the center of a novel network regulating signaling, gene transcription and mRNA translation with remarkable impact on self-renewal, tumor progression and drug resistance (Oncogene 2001; Am J Pathol 2003; Mol Cell Biol 2005; Clin Cancer Res 2008; Oncogene 2014; Mol Cancer 2014; Oncotarget 2017).
More recently, the Thomson lab has characterized new cellular models of progression in prostate, bladder and breast cancers, describing new mechanisms by which cells acquire and/or maintain their self-renewal and metastatic potentials (J Clin Invest 2012, highlighted in Nat Rev Cancer 2012; Mol Cancer 2014; Oncotarget 2017) that represent a refinement of currently held notions on the role played by epithelial and mesenchymal gene programs in the metastatic progression of epithelial cancers.
In close collaboration with M. Cascante, the Thomson lab is currently characterizing the metabolic networks underlying cellular models of tumor progression and acquired resistance to targeted therapies in prostate, colorectal and breast cancers (Stem Cells 2016; Oncotarget 2016; Oncotarget 2017; Mol Syst Biol 2017, PLOS Comput Biol 2018, PLOS One 2018; Front Mol Biosci. 2019; Cancers 2019; J Med Clin 2019).

Monica Pons

Erika Zodda
Past students
Marta Guerra
Antoni Celià
Oscar Meca
Cristina Benítez
Cristina Calvo
Selected publications
Altitude as a protective factor from COVID-19
C1 esterase inhibitor and the contact system in COVID-19.
Thomson TM, Toscano-Guerra E, Casis E, Paciucci R.Br J Haematol. 2020 Jun 12:10.1111/bjh.16938. doi: 10.1111/bjh.16938. Online ahead of print.PMID: 32531085
The Tumor Microenvironment in Colorectal Cancer Therapy.
Pedrosa L, Esposito F, Thomson TM, Maurel J.Cancers (Basel). 2019 Aug 14;11(8):1172. doi: 10.3390/cancers11081172.PMID: 31416205
Metabolic Plasticity and Epithelial-Mesenchymal Transition.
Thomson TM, Balcells C, Cascante M.J Clin Med. 2019 Jul 3;8(7):967. doi: 10.3390/jcm8070967.PMID: 31277295
Metabolic Alterations in Cardiopulmonary Vascular Dysfunction.
Smolders VF, Zodda E, Quax PHA, Carini M, Barberà JA, Thomson TM, Tura-Ceide O, Cascante M.Front Mol Biosci. 2019 Jan 22;5:120. doi: 10.3389/fmolb.2018.00120. eCollection 2018.PMID: 30723719
Epithelial plasticity in cancer: beyond metastasis.
Thomson TM, Fernández PL.Aging (Albany NY). 2018 Jan 22;10(1):3-4. doi: 10.18632/aging.101367.PMID: 29356684
All publications
Altitude as a protective factor from COVID-19
C1 esterase inhibitor and the contact system in COVID-19.
Thomson TM, Toscano-Guerra E, Casis E, Paciucci R.Br J Haematol. 2020 Jun 12:10.1111/bjh.16938. doi: 10.1111/bjh.16938. Online ahead of print.PMID: 32531085
The Tumor Microenvironment in Colorectal Cancer Therapy.
Pedrosa L, Esposito F, Thomson TM, Maurel J.Cancers (Basel). 2019 Aug 14;11(8):1172. doi: 10.3390/cancers11081172.PMID: 31416205
Metabolic Plasticity and Epithelial-Mesenchymal Transition.
Thomson TM, Balcells C, Cascante M.J Clin Med. 2019 Jul 3;8(7):967. doi: 10.3390/jcm8070967.PMID: 31277295
Metabolic Alterations in Cardiopulmonary Vascular Dysfunction.
Smolders VF, Zodda E, Quax PHA, Carini M, Barberà JA, Thomson TM, Tura-Ceide O, Cascante M.Front Mol Biosci. 2019 Jan 22;5:120. doi: 10.3389/fmolb.2018.00120. eCollection 2018.PMID: 30723719
Epithelial plasticity in cancer: beyond metastasis.
Thomson TM, Fernández PL.Aging (Albany NY). 2018 Jan 22;10(1):3-4. doi: 10.18632/aging.101367.PMID: 29356684
Marín de Mas I, Aguilar E, Zodda E, Balcells C, Marin S, Dallmann G, Thomson TM, Papp B, Cascante M.PLoS Comput Biol. 2018 Jan 2;14(1):e1005914. doi: 10.1371/journal.pcbi.1005914. eCollection 2018 Jan.PMID: 29293497
Sánchez-Cid L, Pons M, Lozano JJ, Rubio N, Guerra-Rebollo M, Soriano A, Paris-Coderch L, Segura MF, Fueyo R, Arguimbau J, Zodda E, Bermudo R, Alonso I, Caparrós X, Cascante M, Rafii A, Kang Y, Martínez-Balbás M, Weiss SJ, Blanco J, Muñoz M, Fernández PL, Thomson TM.Oncotarget. 2017 Sep 7;8(48):83384-83406. doi: 10.18632/oncotarget.20698. eCollection 2017 Oct 13.PMID: 29137351
De novo MYC addiction as an adaptive response of cancer cells to CDK4/6 inhibition.
Tarrado-Castellarnau M, de Atauri P, Tarragó-Celada J, Perarnau J, Yuneva M, Thomson TM, Cascante M.Mol Syst Biol. 2017 Oct 4;13(10):940. doi: 10.15252/msb.20167321.PMID: 28978620
A key role for transketolase-like 1 in tumor metabolic reprogramming.
Diaz-Moralli S, Aguilar E, Marin S, Coy JF, Dewerchin M, Antoniewicz MR, Meca-Cortés O, Notebaert L, Ghesquière B, Eelen G, Thomson TM, Carmeliet P, Cascante M.Oncotarget. 2016 Aug 9;7(32):51875-51897. doi: 10.18632/oncotarget.10429.PMID: 27391434
Aguilar E, Marin de Mas I, Zodda E, Marin S, Morrish F, Selivanov V, Meca-Cortés Ó, Delowar H, Pons M, Izquierdo I, Celià-Terrassa T, de Atauri P, Centelles JJ, Hockenbery D, Thomson TM, Cascante M.Stem Cells. 2016 May;34(5):1163-76. doi: 10.1002/stem.2286. Epub 2016 Feb 2.PMID: 27146024
SPARC mediates metastatic cooperation between CSC and non-CSC prostate cancer cell subpopulations.
Mateo F, Meca-Cortés O, Celià-Terrassa T, Fernández Y, Abasolo I, Sánchez-Cid L, Bermudo R, Sagasta A, Rodríguez-Carunchio L, Pons M, Cánovas V, Marín-Aguilera M, Mengual L, Alcaraz A, Schwartz S Jr, Mellado B, Aguilera KY, Brekken R, Fernández PL, Paciucci R, Thomson TM.Mol Cancer. 2014 Oct 21;13:237. doi: 10.1186/1476-4598-13-237.PMID: 25331979
Alaña L, Sesé M, Cánovas V, Punyal Y, Fernández Y, Abasolo I, de Torres I, Ruiz C, Espinosa L, Bigas A, Y Cajal SR, Fernández PL, Serras F, Corominas M, Thomson TM, Paciucci R.Mol Cancer. 2014 Mar 31;13:74. doi: 10.1186/1476-4598-13-74.PMID: 24684754
Infrequent loss of luminal differentiation in ductal breast cancer metastasis.
Calvo J, Sánchez-Cid L, Muñoz M, Lozano JJ, Thomson TM, Fernández PL.PLoS One. 2013 Oct 21;8(10):e78097. doi: 10.1371/journal.pone.0078097. eCollection 2013.PMID: 24205108
Stem cells in prostate cancer.
Mateo F, Fernandez PL, Thomson TM.Arch Esp Urol. 2013 Jun;66(5):475-86.PMID: 23793765 Review.
Regulation of protein translation and c-Jun expression by prostate tumor overexpressed 1.
Marqués N, Sesé M, Cánovas V, Valente F, Bermudo R, de Torres I, Fernández Y, Abasolo I, Fernández PL, Contreras H, Castellón E, Celià-Terrassa T, Méndez R, Ramón Y Cajal S, Thomson TM, Paciucci R.Oncogene. 2014 Feb 27;33(9):1124-34. doi: 10.1038/onc.2013.51. Epub 2013 Mar 4.PMID: 23455324
Acid ceramidase as a therapeutic target in metastatic prostate cancer.
Camacho L, Meca-Cortés O, Abad JL, García S, Rubio N, Díaz A, Celià-Terrassa T, Cingolani F, Bermudo R, Fernández PL, Blanco J, Delgado A, Casas J, Fabriàs G, Thomson TM.J Lipid Res. 2013 May;54(5):1207-20. doi: 10.1194/jlr.M032375. Epub 2013 Feb 19.PMID: 23423838
Zhang S, Zhou Y, Sarkeshik A, Yates JR 3rd, Thomson TM, Zhang Z, Lee EY, Lee MY.J Biol Chem. 2013 Feb 1;288(5):2941-50. doi: 10.1074/jbc.M112.423392. Epub 2012 Dec 11.PMID: 23233665
Nucleolar exit of RNF8 and BRCA1 in response to DNA damage.
Guerra-Rebollo M, Mateo F, Franke K, Huen MS, Lopitz-Otsoa F, Rodríguez MS, Plans V, Thomson TM.Exp Cell Res. 2012 Nov 1;318(18):2365-76. doi: 10.1016/j.yexcr.2012.07.003. Epub 2012 Jul 16.PMID: 22814251
Celià-Terrassa T, Meca-Cortés O, Mateo F, Martínez de Paz A, Rubio N, Arnal-Estapé A, Ell BJ, Bermudo R, Díaz A, Guerra-Rebollo M, Lozano JJ, Estarás C, Ulloa C, Álvarez-Simón D, Milà J, Vilella R, Paciucci R, Martínez-Balbás M, de Herreros AG, Gomis RR, Kang Y, Blanco J, Fernández PL, Thomson TM.J Clin Invest. 2012 May;122(5):1849-68. doi: 10.1172/JCI59218. Epub 2012 Apr 16.PMID: 22505459
Bermudo R, Abia D, Mozos A, García-Cruz E, Alcaraz A, Ortiz AR, Thomson TM, Fernández PL.Br J Cancer. 2011 Nov 8;105(10):1600-7. doi: 10.1038/bjc.2011.435. Epub 2011 Oct 18.PMID: 22009027
Differential regulation of RNF8-mediated Lys48- and Lys63-based poly-ubiquitylation.
Lok GT, Sy SM, Dong SS, Ching YP, Tsao SW, Thomson TM, Huen MS.Nucleic Acids Res. 2012 Jan;40(1):196-205. doi: 10.1093/nar/gkr655. Epub 2011 Sep 12.PMID: 21911360
Bermudo R, Abia D, Benitez D, Carrió A, Vilella R, Ortiz AR, Thomson TM, Fernández PL.Ann N Y Acad Sci. 2010 Oct;1210:17-24. doi: 10.1111/j.1749-6632.2010.05780.x.PMID: 20973795
Protein-protein interaction antagonists as novel inhibitors of non-canonical polyubiquitylation.
Scheper J, Guerra-Rebollo M, Sanclimens G, Moure A, Masip I, González-Ruiz D, Rubio N, Crosas B, Meca-Cortés O, Loukili N, Plans V, Morreale A, Blanco J, Ortiz AR, Messeguer A, Thomson TM.PLoS One. 2010 Jun 30;5(6):e11403. doi: 10.1371/journal.pone.0011403.PMID: 20613989
Monoubiquitination of RPN10 regulates substrate recruitment to the proteasome.
Isasa M, Katz EJ, Kim W, Yugo V, González S, Kirkpatrick DS, Thomson TM, Finley D, Gygi SP, Crosas B.Mol Cell. 2010 Jun 11;38(5):733-45. doi: 10.1016/j.molcel.2010.05.001.PMID: 20542005
Regulation of aurora B kinase by the lipid raft protein flotillin-1.
Gómez V, Sesé M, Santamaría A, Martínez JD, Castellanos E, Soler M, Thomson TM, Paciucci R.J Biol Chem. 2010 Jul 2;285(27):20683-90. doi: 10.1074/jbc.M110.130591. Epub 2010 Apr 29.PMID: 20430883
Ubiquitin and SUMO signalling in DNA repair.
Thomson TM, Guerra-Rebollo M.Biochem Soc Trans. 2010 Feb;38(Pt 1):116-31. doi: 10.1042/BST0380116.PMID: 20074046 Review.
Crosas B, Farràs R, Marfany G, Rodríguez MS, Thomson TM. Biochem Soc Trans. 2010 Feb;38(Pt 1):1-5. doi: 10.1042/BST0380001.PMID: 20074026
Soler M, Mancini F, Meca-Cortés O, Sánchez-Cid L, Rubio N, López-Fernández S, Lozano JJ, Blanco J, Fernández PL, Thomson TM. Int J Cancer vol. 125, 2565-2575 (2009). doi: 10.1002/ijc.24651
Bermudo R, Abia D, Ferrer B, Nayach I, Benguria A, Zaballos A, del Rey J, Miró R, Campo E, Martínez-A C, Ortiz AR, Fernández PL, Thomson TM. BMC Cancer vol. 8, 315 (2008). doi: 10.1186/1471-2407-8-315
Scheper J, Oliva B, Villà-Freixa J, Thomson TM. Proteins vol. 74, 92 – 103 (2008). doi: 10.1002/prot.22120
Regulation of mitotic exit by the RNF8 ubiquitin ligase.
Plans V, Guerra-Rebollo M, Thomson TM (2008). Oncogene vol. 27, 1355 – 1365 (2008). doi: 10.1038/sj.onc.1210782
Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase.
Kolas NK, Chapman JR, Nakada S, Ylanko J, Chahwan R, Sweeney FD, Panier S, Mendez M, Wildenhain J, Thomson TM, Pelletier L, Jackson SP, Durocher D. Science vol. 318, 1637 – 1640 (2007). doi: 10.1126/science.1150034
The RING finger protein RNF8 recruits UBC13 for lysine 63-based self polyubiquitylation.
Plans V, Scheper J, Soler M, Loukili N, Okano Y, Thomson TM. J Cell Biochem vol. 97, 572 – 582 (2006). doi: 10.1002/jcb.20587
Lozano JJ, Soler M, Bermudo R, Abia D, Fernandez PL, Thomson TM, Ortiz AR. BMC Genomics vol. 6, 1 – 18 (2005). doi: 10.1186/1471-2164-6-109
Ferrer B, Bermudo R, Thomson T, Nayach I, Soler M, Sanchez M, Castillo M, Calvo J, Campo E, Fernandez PL. Pathobiology vol. 72, 225 – 232 (2005). doi: 10.1159/000089416
Diaz VM, Hurtado M, Kort EJ, Resnati M, Blasi F, Thomson T, Paciucci R. Experimental Cell Research vol. 312, 2203 – 2213 (2006). doi: 10.1016/j.yexcr.2006.03.017
Diaz VM, Hurtado M; Thomson TM, Reventos J, Paciucci R. Gut vol. 53, 993 – 1000 (2004). doi: 10.1136/gut.2003.026831
Diaz VM, Planaguma J, Thomson TM, Reventos J, Paciucci R. Gastroenterology vol. 122, 806-819 (2003). doi: 10.1053/gast.2002.31885
Project funding
Nuevos métodos de cribaje combinado, experimental y virtual, para la identificación y desarrollo de pequeñas moléculas de interferencia específica de interacciones proteína-proteína y desacoplamiento de rutas de señalización
- AGENCY: CSIC
- PERIOD: 11/2005 – 10/2007
- PI: Timothy M Thomson
Genes de malignidad en cáncer de próstata: validación de PTOV1 como diana terapéutica mediante administración in vivo de siRNA e identificación no sesgada de determinantes moleculares de metástasis
- AGENCY: Programa Nacional de Biomedicina, MEC
- PERIOD: 12/2005 – 12/2008
- PI: Timothy M Thomson
New molecules in the diagnosis and therapy of prostate and breast cancers. Modulation of tumor growth by the chemokine CX3CL1 (Fractalkine)
- AGENCY: Fundació La Marató de TV3
- PERIOD: 12/2005 – 12/2008
- PI: Timothy M Thomson
Nuevos marcadores de malignidad identificados mediante procedimientos de alto rendimiento. Desarrollo y demostración de nuevas plataformas para el diagnóstico molecular de cáncer de próstata
- AGENCY: PROG. DE ESTIMULO A LA TRANS. DE RESULTADOS DE INVESTIGACION, MEC
- PERIOD: 12/2006 – 12/2008
- PI: Timothy M Thomson
Regulation of gene networks common to cancer stem cells and malignant progression
- AGENCY: Spanish Ministry of Education and Science
- PERIOD: 01/2009 – 12/2011
- PI: Timothy M Thomson
Estudio fenotípico de células madre neoplásicas y de su transición epitelio-mesénquima en muestras exvivo derivadas de adenocarcinomas prostáticos humanos.
- AGENCY: Spanish Ministry of Foreign Affairs (AECID)
- PERIOD: 01/2009 – 12/2009
- PI: Timothy M Thomson
Desarrollo preclínico de nuevos compuestos antitumorales
- AGENCY: Generalitat de Catalunya (CIDEM)
- PERIOD: 06/2009 – 06/2010
- PI: Timothy M. Thomson
Grupo reconocido y financiado por la Generalitat de Catalunya “Cáncer, señalización y ubiquitinación”
- AGENCY: Generalitat de Catalunya AGAUR SGR-DGR
- PERIOD: 2009 – 2014
- PI: Timothy M Thomson
Vacancies/Jobs
If you are interested in joining the lab as postdoc or PhD student please send us your CV and cover letter: Timothy Thomson (titbmc@ibmb.csic.es).