Lab presentation
Our group is interested in unveiling the molecular mechanisms supporting endocytic traffic, with a particular focus on the characterization of actin scaffolds that transiently assemble at the plasma or endosomal membranes to aid budding and fission. We investigate the components of these endocytic actin scaffolds, the physico-chemical parameters that regulate their assembly and turn over, and their particular architecture and coupling to membranes. All these parameters define the geometry and the size of the membrane limited transport intermediates and coordinate membrane budding with cargo loading.
To this end, we mostly use S. cerevisiae as a model system, but we also extend our investigations to mammalian cells. By combining the powerful yeast genetics or the CRISPR CAS9 technology in mammalian cells, together with actin polymerization in vitro assays, live-cell fluorescence imaging of single endocytic events and sophisticated time-resolved ultrastructural analysis, we provide data to refine molecular models explaining membrane deformation, and we uncover mechanisms regulating actin dynamics.
Our research has led to the discovery of new functions or regulators of conserved proteins whose mutation in humans cause inherited renal, immunological and neurological diseases such as focal segmental glomerulosclerosis (myo IE, ORPHA93213), the Wiskott–Aldrich syndrome (N-WASP, ORPHA906), amyotrophic lateral sclerosis (VAPs, ORPs, ORPHA209335), atypical juvenile parkinsonism (SNJ1, ORPHA:391411) or autosomal dominant non-syndromic intellectual disability (CKB1, ORPHA:178469). This information is key to understand their role in health and disease and has identified potential therapeutic targets to ameliorate the symptoms of individuals that bear mutations in these genes.
Projects
Research Lines:
- Molecular mechanisms of endocytic traffic:
Protein and membrane traffic throughout the endocytic pathway requires a core machinery effecting membrane budding, vesicle and organelle motility and membrane fusion, which is conserved from yeast to humans. We aim to identify the minimal set of evolutionary-conserved proteins required to support some of these events (with a particular focus in membrane budding), and to understand how their transient assembly is regulated to effect membrane deformation. IN the last years we have focus in understanding the interplay between the actin cytoskeleton components and different biologically active lipid species including sterols, phosphoinositides and ceramides. Further, we investigate the accessory endocytic proteins only expressed in higher eukaryotes, which adapt the endocytic core machinery to the more complex physiological functions in multicellular organisms.
Projects:
- Function and regulation of ER-endocytic contact sites and the mechanism of sterol-induced actin polymerization.
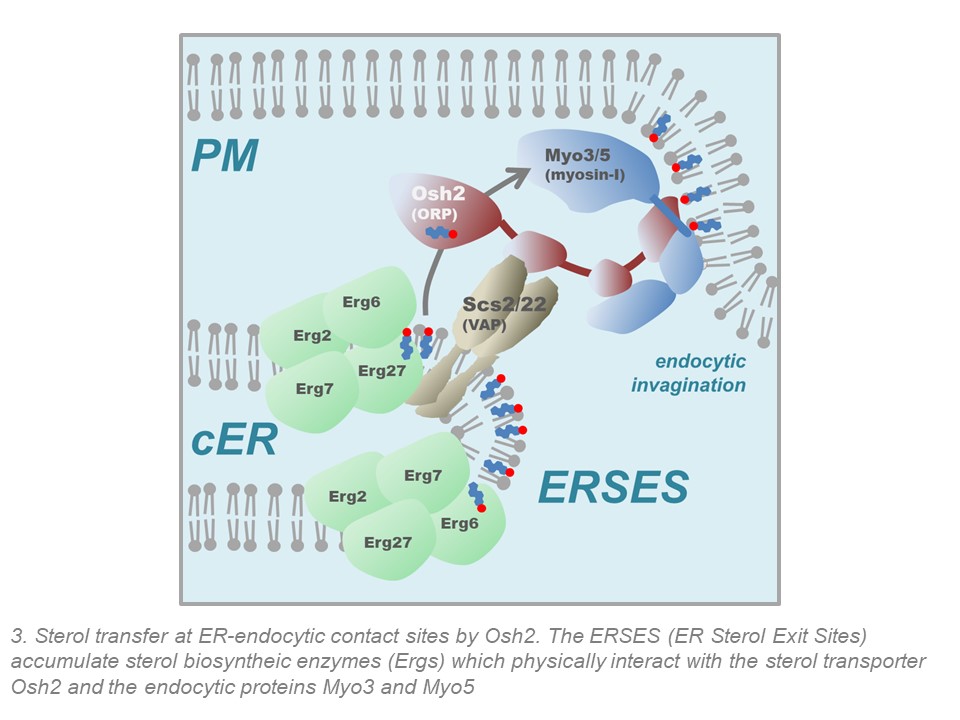
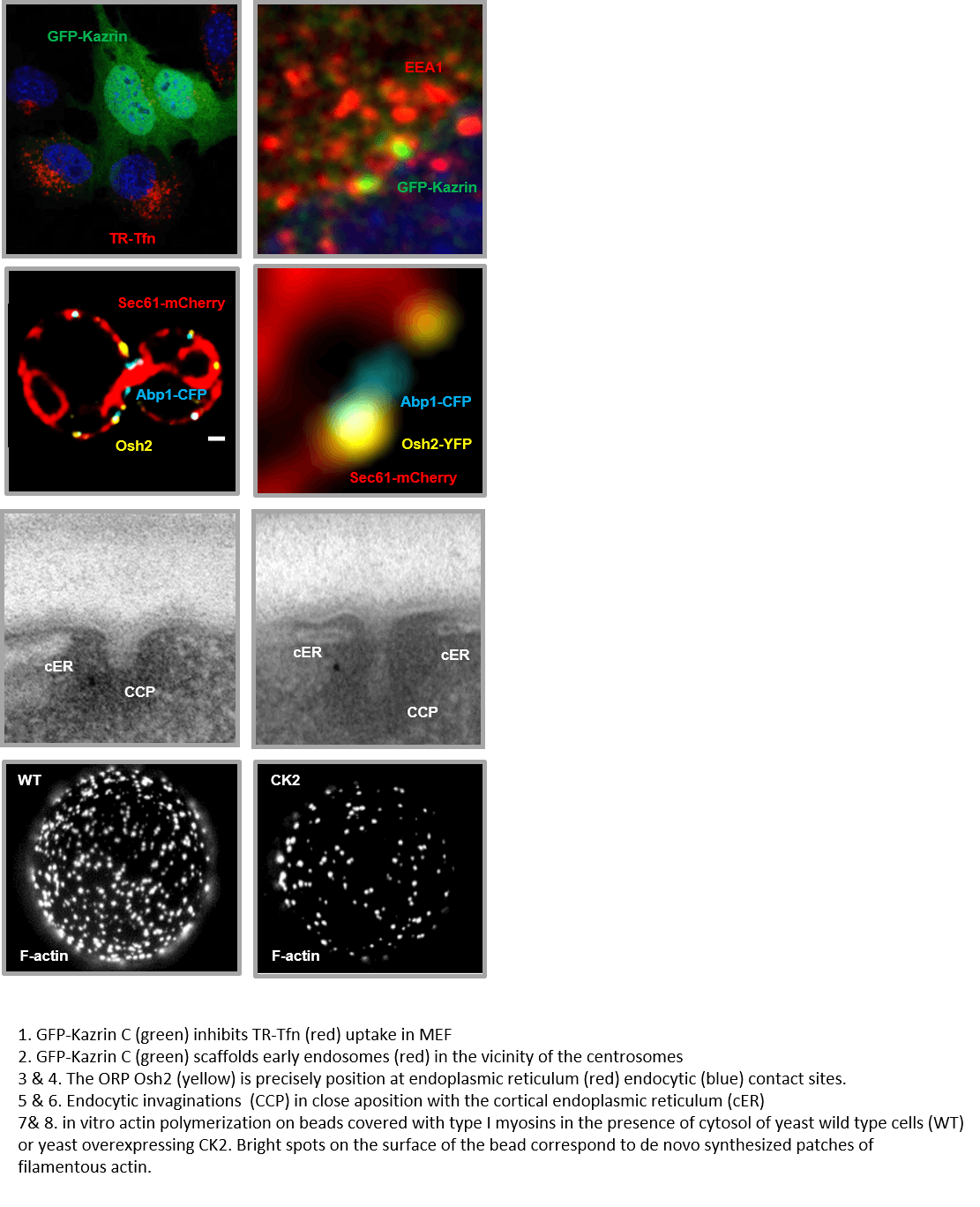
We previously demonstrated that the endoplasmic reticulum (ER) contacts the endocytic sites to facilitate actin polymerization and membrane invagination during endocytic uptake. We found that the molecular link between the cER and the endocytic sites is composed of the yeast homologs of the human VAP (VAMP Associated Proteins) A and B, Oxysterol binding protein Related Proteins (ORPs) and the endocytic type I myosins (Encinar del Dedo et al Dev. Cell (2017)). Our data indicate that the VAP/ORP/Myosin-I complex has the capacity to transfer sterols to the plasma membrane from spezialized ER subdomains where sterol synthesis and transport are physically coupled. We have baptized such subdomains as ERSES (ER Sterol Exit Sites) (Encinar del Dedo et al J Cell Biol (2021)). We also found that this coupled sterol synthesis and transfer activity is specifically required to concentrate and activate the type I myosins, which in turn initiate actin polymerization at endocytic sites. We are now in the process of characterizing the molecular link between the sterols and the recruitment and activation of actin nucleator promoting factors, which might involve the activation of the PI4P-5 kinase Mss4, the sterol-dependent activation of calcium channels, the recruitment of small GTPases and/or the formation of lipid nano-subdomains. We are also currently characterizing the components of the ERSES and defining its function and regulation.
- Transient assembly of macromomolecular complexes; regulation by phosphoinositides and CK2.
We previously found that the yeast synaptojanins, which hydrolyze the 4 and 5 phosphates of PIns(4,5)P2, cooperate with the promiscuous casein kinase 2 (CK2) to disassemble the actin scaffold induced by N-WASP and type I myosins at endocytic sites (Fernández-Golbano (2014) Dev Cell). Disassembly occurs in a very narrow time window, indicating that a mechanism coordinating the synaptojanin and CK2 activities might exist. Interestingly, we found that different phosphoinositides bind and inhibit CK2 in vitro, so that its activity towards endocytic proteins might only be triggered upon hydrolysis of PIns(4,5)P2 or PI4P by the synaptojanins. What is the physiological relevance of this regulatory crosstalk in vivo remained to be demonstrated. Using structural approaches in collaboration with the group of N. Verdaguer at the IBMB, we have identified the phosphoinosite binding site in CK2 and demonstrated its functional relevance in vivo. Further, a proteomic screen in collaboration with the group of S. Gygi at Harvard University has unveiled other cellular process regulated by the phosphoinositide/CK2 regulatory loop, including membrane budding from Golgi membranes, exocytosis or mechanosensing.
- A multivalent regulator of endosomal traffic in mammalian cells that regulates actin polymerization and tubulin-dependent transport.
We identified kazrin C as a human protein that inhibits clathrin-mediated endocytosis when overexpressed. We have generated kazrin knock out and GFP-kazrin C knock in mouse embryonic fibroblast to investigate its function in endocytic trafficking. We found that kazrin depletion causes a defect in traffic from early (EE) to recycling endosomes (RE). Cellular functions that require unaltered endocytic recycling, such as cell migration or cytokinetic abscission, are also affected in the knock out cells. Consistent with its function, endogenous kazrin is detected on EE and interacts with several components of these organelles, including Epsin Homology Domain proteins EHD1 and EHD3, gamma adaptin and phosphatidyl-inositol-3 phosphate. In vivo, we found that overexpression of GFP-kazrin C forms a condensate that entraps EE in the vicinity of the centromere, suggesting that kazrin is either an endosomal adaptor for dynein or it retains dynein cargo in the pericentriolar region. Further, we found that kazrin inhibits actin polymerization on EE membranes.The data thus suggest that kazrin is a multivalent regulator of endocytic recycling that promotes microtubule dependent traffic or retention of EE at the perinuclear region, while it downregulates endosomal actin polymerization. We are currently conducting experiments to define the actin nucleating promoting factors directly regulated by kazrin, and define its interaction with dynein and the dynactin complex.
Lab people

Mª Isabel Geli Fernández-Peñaflor
Principal investigator
After her Ph. D. in plant cell biology, M. I. Geli moved to the laboratory of H. Riezman at the Biozentrum of the University of Basel, where she contributed to identify several proteins involved in endocytosis, including the unconventional type I myosins (Geli and Riezman 1996 Science). In January 1999, M. I. Geli started her scientific career as an independent researcher at the Biochemie-Zentrum of the University of Heidelberg, where she established a research line to understand how the transient assembly of macromolecular complexes can drive deformation of cellular membranes. In 2003, she got a tenior position as Científico Titular of the CSIC at the Instituto de Biología Molecular de Barcelona, where she was promoted to Investigador Científico in 2009.
Along her carrier as principal investigator, M. I. Geli has made significant contributions to the field, which include the description of the molecular mechanisms regulating type I myosins and actin polymerization (Geli et al 1998 EMBO J, and Grötsch et al 2010 EMBO J); the development of time-resolved electron microscopy to study endocytic budding with unprecedented resolution (Idrissi et al 2008 J Cell Biol and Idrissi et al 2010 PNAS); the description a phosphoinositide/CK2 interplay (Fernandez-Golbano et al 2014 Dev. Cell.); the discovery and functional characterization of the ER endocytic contact sites (Encinar del Dedo et al., 2017 Dev Cell), and the identification of a new endoplasmic reticulum subdomain, the ERSES (ER Sterol Exit Sites), where synthesis an export of sterols are coordinated (Encinar del Dedo et al 2020 J Cell Biol). Since 2014, M. I. Geli is Deputy Director of the IBMB, and since 2020 she is member of the Editorial Board of eLife.

Laura Pastor

Isabel María Fernández Golbano
Past students
Inés Hernández Pérez
Javier Encinar del Dedo
Isabel M. Fernández
Adrian Baumann
Fatima Idrissi
Jonathan Giblin
Henrique Girao
Virginia Robles Garcia
Maria del Mar Borrás Quintanal
Helga Grötsch
Birgit Schmelzl
Bianka Wolf
Selected publications
Encinar Del Dedo, J., Fernandez-Golbano, I.M., Pastor, L., Meler, P., Ferrer-Orta, C., Rebollo, E., and Geli, M.I. (2021). Coupled sterol synthesis and transport machineries at ER-endocytic contact sites. The Journal of Cell Biology 220. doi: 10.1083/jcb.202010016
Encinar Del Dedo, J., Idrissi, F.Z., Fernandez-Golbano, I.M., Garcia, P., Rebollo, E., Krzyzanowski, M.K., Grotsch, H., and Geli, M.I. (2017). ORP-Mediated ER contact with endocytic sites facilitates actin polymerization. Developmental Cell 43, 588-602 e586. doi: 10.1016/j.devcel.2017.10.031
Fernandez-Golbano, I.M., Idrissi, F.Z., Giblin, J.P., Grosshans, B.L., Robles, V., Grotsch, H., Borras Mdel, M., and Geli, M.I. (2014). Crosstalk between PI(4,5)P(2)and CK2 modulates actin polymerization during endocytic uptake. Developmental Cell 30, 746-758. doi: 10.1016/j.devcel.2014.07.020
Idrissi, F.Z., Blasco, A., Espinal, A., and Geli, M.I. (2012). Ultrastructural dynamics of proteins involved in endocytic budding. Proceedings of the National Academy of Sciences of the United States of America 109, E2587-2594. doi: 10.1073/pnas.1202789109
Grotsch, H., Giblin, J.P., Idrissi, F.Z., Fernandez-Golbano, I.M., Collette, J.R., Newpher, T.M., Robles, V., Lemmon, S.K., and Geli, M.I. (2010). Calmodulin dissociation regulates Myo5 recruitment and function at endocytic sites. The EMBO Journal 29, 2899-2914. doi: 10.1073/pnas.1202789109
Idrissi, F.Z., Grotsch, H., Fernandez-Golbano, I.M., Presciatto-Baschong, C., Riezman, H., and Geli, M.I. (2008). Distinct acto/myosin-I structures associate with endocytic profiles at the plasma membrane. The Journal of Cell Biology 180, 1219-1232. doi: 10.1083/jcb.200708060
Geli, M.I., Lombardi, R., Schmelzl, B., and Riezman, H. (2000). An intact SH3 domain is required for myosin I-induced actin polymerization. The EMBO Journal 19, 4281-4291. doi: 10.1093/emboj/19.16.4281
Geli, M.I., Wesp, A., and Riezman, H. (1998). Distinct functions of calmodulin are required for the uptake step of receptor-mediated endocytosis in yeast: the type I myosin Myo5p is one of the calmodulin targets. The EMBO Journal 17, 635-647. doi: 10.1093/emboj/17.3.635
Geli, M.I., and Riezman, H. (1996). Role of type I myosins in receptor-mediated endocytosis in yeast. Science 272, 533-535. https://www.jstor.org/stable/2890100
Geli, M.I., Torrent, M., and Ludevid, D. (1994). Two Structural Domains Mediate Two Sequential Events in [gamma]-Zein Targeting: Protein Endoplasmic Reticulum Retention and Protein Body Formation. The Plant Cell 6, 1911-1922. doi: 10.1105/tpc.6.12.1911
All publications
Encinar Del Dedo, J., Fernandez-Golbano, I.M., Pastor, L., Meler, P., Ferrer-Orta, C., Rebollo, E., and Geli, M.I. (2021). Coupled sterol synthesis and transport machineries at ER-endocytic contact sites. The Journal of Cell Biology 220. doi: 10.1083/jcb.202010016
Garcia, P., Coll, P.M., Del Rey, F., Geli, M.I., Perez, P., Vazquez de Aldana, C.R., and Encinar Del Dedo, J. (2021). Eng2, a new player involved in feedback loop regulation of Cdc42 activity in fission yeast. Scientific Reports 11, 17872. doi: 10.1038/s41598-021-97311-6
Pons, M., Izquierdo, I., Andreu-Carbo, M., Garrido, G., Planaguma, J., Muriel, O., Del Pozo, M.A., Geli, M.I., and Aragay, A.M. (2017). Phosphorylation of filamin A regulates chemokine receptor CCR2 recycling. Journal of Cell Science 130, 490-501. doi: 10.1242/jcs.193821
Encinar Del Dedo, J., Idrissi, F.Z., Fernandez-Golbano, I.M., Garcia, P., Rebollo, E., Krzyzanowski, M.K., Grotsch, H., and Geli, M.I. (2017). ORP-Mediated ER contact with endocytic sites facilitates actin polymerization. Developmental Cell 43, 588-602 e586. doi: 10.1016/j.devcel.2017.10.031
Amaral, N., Vendrell, A., Funaya, C., Idrissi, F.Z., Maier, M., Kumar, A., Neurohr, G., Colomina, N., Torres-Rosell, J., Geli, M.I., et al. (2016). The Aurora-B-dependent NoCut checkpoint prevents damage of anaphase bridges after DNA replication stress. Nature Cell Biology 18, 516-526. doi: 10.1038/ncb3343
Encinar del Dedo, J., Idrissi, F.Z., Arnaiz-Pita, Y., James, M., Duenas-Santero, E., Orellana-Munoz, S., del Rey, F., Sirotkin, V., Geli, M.I., and Vazquez de Aldana, C.R. (2014). Eng2 is a component of a dynamic protein complex required for endocytic uptake in fission yeast. Traffic15, 1122-1142. doi: 10.1111/tra.12198
Fernandez-Golbano, I.M., Idrissi, F.Z., Giblin, J.P., Grosshans, B.L., Robles, V., Grotsch, H., Borras Mdel, M., and Geli, M.I. (2014). Crosstalk between PI(4,5)P(2)and CK2 modulates actin polymerization during endocytic uptake. Developmental Cell 30, 746-758. doi: 10.1016/j.devcel.2014.07.020
Idrissi, F.Z., and Geli, M.I. (2014). Zooming in on the molecular mechanisms of endocytic budding by time-resolved electron microscopy. Cellular and Molecular Life Sciences : CMLS 71, 641-657. doi: 10.1007/s00018-013-1452-8
Herms, A., Bosch, M., Ariotti, N., Reddy, B.J., Fajardo, A., Fernandez-Vidal, A., Alvarez-Guaita, A., Fernandez-Rojo, M.A., Rentero, C., Tebar, F., et al. (2013). Cell-to-cell heterogeneity in lipid droplets suggests a mechanism to reduce lipotoxicity. Current Biology : CB 23, 1489-1496. doi: 10.1016/j.cub.2013.06.032
Idrissi, F.Z., Blasco, A., Espinal, A., and Geli, M.I. (2012). Ultrastructural dynamics of proteins involved in endocytic budding. Proceedings of the National Academy of Sciences of the United States of America 109, E2587-2594. doi: 10.1073/pnas.1202789109
Giblin, J., Fernandez-Golbano, I.M., Idrissi, F.Z., and Geli, M.I. (2011). Function and regulation of Saccharomyces cerevisiae myosins-I in endocytic budding. Biochemical Society transactions 39, 1185-1190. doi: 10.1042/BST0391185
Grotsch, H., Giblin, J.P., Idrissi, F.Z., Fernandez-Golbano, I.M., Collette, J.R., Newpher, T.M., Robles, V., Lemmon, S.K., and Geli, M.I. (2010). Calmodulin dissociation regulates Myo5 recruitment and function at endocytic sites. The EMBO Journal 29, 2899-2914. doi: 10.1073/pnas.1202789109
Collette, J.R., Chi, R.J., Boettner, D.R., Fernandez-Golbano, I.M., Plemel, R., Merz, A.J., Geli, M.I., Traub, L.M., and Lemmon, S.K. (2009). Clathrin functions in the absence of the terminal domain binding site for adaptor-associated clathrin-box motifs. Molecular Biology of the Cell 20, 3401-3413. doi: 10.1091/mbc.e08-10-1082
Idrissi, F.Z., Grotsch, H., Fernandez-Golbano, I.M., Presciatto-Baschong, C., Riezman, H., and Geli, M.I. (2008). Distinct acto/myosin-I structures associate with endocytic profiles at the plasma membrane. The Journal of Cell Biology 180, 1219-1232. doi: 10.1083/jcb.200708060
Girao, H., Geli, M.I., and Idrissi, F.Z. (2008). Actin in the endocytic pathway: from yeast to mammals. FEBS letters 582, 2112-2119. doi: 10.1016/j.febslet.2008.04.011
De Luca, A.C., Volpe, G., Drets, A.M., Geli, M.I., Pesce, G., Rusciano, G., Sasso, A., and Petrov, D. (2007). Real-time actin-cytoskeleton depolymerization detection in a single cell using optical tweezers. Optics Express 15, 7922-7932. doi: 10.1364/OE.15.007922
Newpher, T.M., Idrissi, F.Z., Geli, M.I., and Lemmon, S.K. (2006). Novel function of clathrin light chain in promoting endocytic vesicle formation. Molecular Biology of the Cell 17, 4343-4352. doi: 10.1091/mbc.e06-07-0606
Grosshans, B.L., Grotsch, H., Mukhopadhyay, D., Fernandez, I.M., Pfannstiel, J., Idrissi, F.Z., Lechner, J., Riezman, H., and Geli, M.I. (2006). TEDS site phosphorylation of the yeast myosins I is required for ligand-induced but not for constitutive endocytosis of the G protein-coupled receptor Ste2p. Journal of Biological Chemistry 281, 11104-11114. doi: 10.1074/jbc.M508933200
Chang, J.S., Henry, K., Geli, M.I., and Lemmon, S.K. (2006). Cortical recruitment and nuclear-cytoplasmic shuttling of Scd5p, a protein phosphatase-1-targeting protein involved in actin organization and endocytosis. Molecular Biology of the Cell 17, 251-262. doi: 10.1091/mbc.e05-10-0936
Schmelzl, B., and Geli, M.I. (2002). An efficient genetic screen in mammalian cultured cells. EMBO Reports 3, 682-687. doi: 10.1093/embo-reports/kvf131
Chang, J.S., Henry, K., Wolf, B.L., Geli, M., and Lemmon, S.K. (2002). Protein phosphatase-1 binding to scd5p is important for regulation of actin organization and endocytosis in yeast. Journal of Biological Chemistry 277, 48002-48008. doi: 10.1074/jbc.M208471200
Idrissi, F.Z., Wolf, B.L., and Geli, M.I. (2002). Cofilin, but not profilin, is required for myosin-I-induced actin polymerization and the endocytic uptake in yeast. Molecular Biology of the Cell 13, 4074-4087. doi: 10.1091/mbc.02-04-0052
Geli, M.I., Lombardi, R., Schmelzl, B., and Riezman, H. (2000). An intact SH3 domain is required for myosin I-induced actin polymerization. The EMBO Journal 19, 4281-4291. doi: 10.1093/emboj/19.16.4281
Munn, A.L., Stevenson, B.J., Geli, M.I., and Riezman, H. (1995). end5, end6, and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Molecular Biology of the Cell 6, 1721-1742. doi: 10.1091/mbc.6.12.1721
Alvarez, I., Geli, M.I., Pimentel, E., Ludevid, D., and Torrent, M. (1998). Lysine-rich gamma-zeins are secreted in transgenic Arabidopsis plants. Planta 205, 420-427. doi: 10.1007/s004250050339
Geli, M.I., and Riezman, H. (1998). Endocytic internalization in yeast and animal cells: similar and different. Journal of Cell Science 111 ( Pt 8), 1031-1037. doi: 10.1242/jcs.111.8.1031
Geli, M.I., Wesp, A., and Riezman, H. (1998). Distinct functions of calmodulin are required for the uptake step of receptor-mediated endocytosis in yeast: the type I myosin Myo5p is one of the calmodulin targets. The EMBO Journal 17, 635-647. doi: 10.1093/emboj/17.3.635
Geli, M.I., and Riezman, H. (1996). Role of type I myosins in receptor-mediated endocytosis in yeast. Science 272, 533-535. https://www.jstor.org/stable/2890100
Torrent, M., Alvarez, I., Geli, M.I., Dalcol, I., and Ludevid, D. (1997). Lysine-rich modified gamma-zeins accumulate in protein bodies of transiently transformed maize endosperms. Plant Molecular Biology 34, 139-149. doi: 10.1023/A:1005889314967
Riezman, H., Munn, A., Geli, M.I., and Hicke, L. (1996). Actin-, myosin- and ubiquitin-dependent endocytosis. Experientia 52, 1033-1041. doi: 10.1007/BF01952099
Geli, M.I., Torrent, M., and Ludevid, D. (1994). Two Structural Domains Mediate Two Sequential Events in [gamma]-Zein Targeting: Protein Endoplasmic Reticulum Retention and Protein Body Formation. The Plant Cell 6, 1911-1922. doi: 10.1105/tpc.6.12.1911
Torrent, M., Geli, M.I., Ruiz-Avila, L., Canals, J.M., Puigdomenech, P., and Ludevid, D. (1994). Role of structural domains for maize gamma-zein retention in Xenopus oocytes. Planta 192, 512-518. doi: 10.1007/BF00203589
Project funding
Main National Grants
PID2020-120053GB-I00
Title: Regulation of transient macromolecular complexes by sterols, phosphoinositides and CK2
Principal Investigator: María Isabel Geli
Granting Agency: SECRETARÍA DE ESTADO DE INVESTIGACIÓN, DESARROLLO E INNOVACIÓN
Duration: 01/09/21 – 30/08/2024
Proyecto PID2020-120053GB-I00 financiado por:

BFU2017-82959-P
Title: MOLECULAR MECHANISMS OF ENDOCYTIC TRAFFIC
Principal Investigator: María Isabel Geli
Granting Agency: SECRETARÍA DE ESTADO DE INVESTIGACIÓN, DESARROLLO E INNOVACIÓN
Duration: 01/01/18 – 30/06/2020
Proyecto BFU2017-82959-P financiado por:

BFU2014-59765-P
Title: MOLECULAR MECHANISMS OF ENDOCYTIC TRAFFIC
Principal Investigator: María Isabel Geli
Granting Agency: SECRETARIA DE ESTADO DE INVESTIGACIÓN, DESARROLLO E INNOVACIÓN
Duration: 01/01/2015 – 31/12/2017
BFU2011-30185
Title: Mechanism and physiological functions of endocytosis
Principal Investigator: María Isabel Geli Fernández-Peñaflor
Granting Agency: SECRETARIA DE ESTADO DE INVESTIGACIÓN, DESARROLLO E INNOVACIÓN Duration: 01/01/2012 – 31/12/2014
CSD2009-00016
Title: Mechanism of Protein Secretion and Compartment Organization
Principal Investigator: María Isabel Geli
Coordinador Prof. Vivek Malhortra (CRG, Barcelona)
Granting Agency: SECRETARIA DE ESTADO DE INVESTIGACIÓN, DESARROLLO E INNOVACIÓN Duration: 01/01/2010 – 31/12/2014
BFU2008-03500
Title: ANalysis of endocytic uptake associated to lipid rafts
Principal Investigator: María Isabel Geli
Granting Agency: SECRETARIA DE ESTADO DE INVESTIGACIÓN, DESARROLLO E INNOVACIÓN Duration: 01/01/2009 – 31/12/2011
BFU2005-04089
Title: Analysis of ubiquitin, sterol and actin-dependent endocytic internalization of a G-protein associated receptor (GPCR) in yeast
Principal Investigator: María Isabel Geli
Granting Agency: SECRETARIA DE ESTADO DE INVESTIGACIÓN, DESARROLLO E INNOVACIÓN Duration: 01/01/2006 – 31/12/2008
SAF2002-04707
Title: ANalysis of endocytic uptake associated to lipid rafts and actin
Principal Investigator: María Isabel Geli
Granting Agency: SECRETARIA DE ESTADO DE INVESTIGACIÓN, DESARROLLO E INNOVACIÓN Duration: 01/01/2003 – 31/12/2005
International Grants
SFB 352 (C10)
Title: Molecular Mechanism of Intracellular Transport Processes
Principal Investigator: María Isabel Geli
Granting Agency: Deutsche Forschunggemeinschaft
Duration: 01/01/1999 – 31/12/2003
SPP1068
Title: Molecular MOTORS
Principal Investigator: María Isabel Geli
Granting Agency: Deutsche Forschunggemeinschaft
Duration: 01/01/2000 – 31/12/2000
Vacancies/Jobs
If you are interested in joining the lab as postdoc or PhD student please send us your CV and cover letter: Maribel Geli (mgfbmc@ibmb.csic.es).
Project gallery
No albums or photos uploaded yet.