The Chromatin and Gene Regulation Group, led by Dr. Guillermo P. Vicent at IBMB, has…
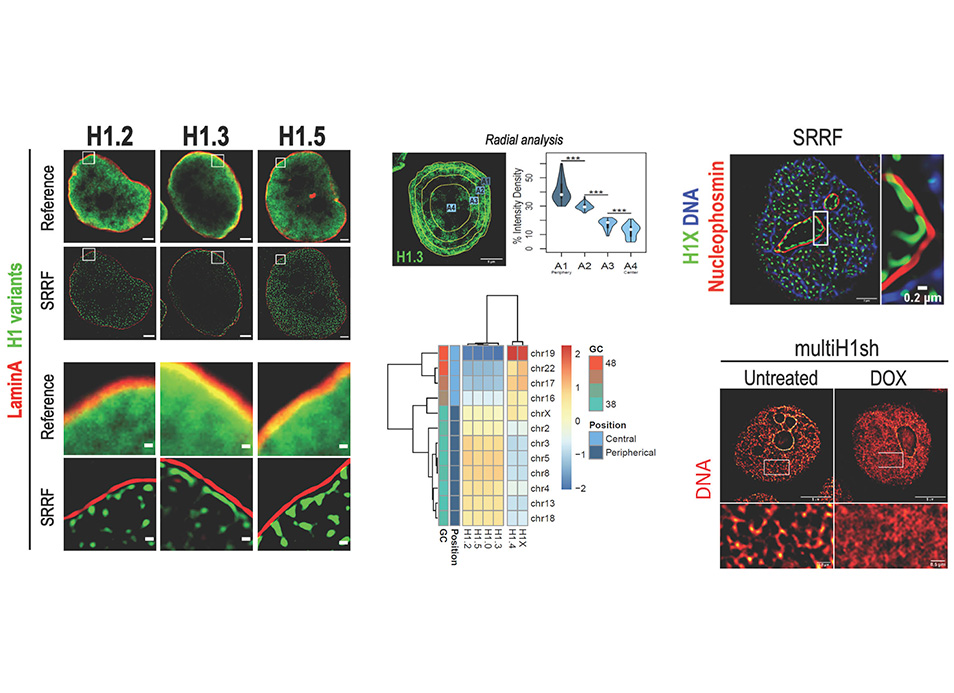
New publication in eLife of the Jordan Lab in collaboration with the IBMB Imaging Platform showing that some human histone H1 variants are universally enriched at the nuclear periphery close to lamin while others are distributed throughout the nuclei, with the particularity of H1X being enriched within the nucleoli, with putative distinct participation on chromatin and nuclear structure organisation.
New publication in eLife of the Jordan Lab in collaboration with the IBMB Imaging Platform showing that some human histone H1 variants are universally enriched at the nuclear periphery close to lamin while others are distributed throughout the nuclei, with the particularity of H1X being enriched within the nucleoli, with putative distinct participation on chromatin and nuclear structure organisation.
https://elifesciences.org/
Abstract
Histone H1 participates in chromatin condensation and regulates nuclear processes. Human somatic cells may contain up to seven histone H1 variants, although their functional heterogeneity is not fully understood. Here, we have profiled the differential nuclear distribution of the somatic H1 repertoire in human cells through imaging techniques including super-resolution microscopy. H1 variants exhibit characteristic distribution patterns in both interphase and mitosis. H1.2, H1.3, and H1.5 are universally enriched at the nuclear periphery in all cell lines analyzed and co-localize with compacted DNA. H1.0 shows a less pronounced peripheral localization, with apparent variability among different cell lines. On the other hand, H1.4 and H1X are distributed throughout the nucleus, being H1X universally enriched in high-GC regions and abundant in the nucleoli. Interestingly, H1.4 and H1.0 show a more peripheral distribution in cell lines lacking H1.3 and H1.5. The differential distribution patterns of H1 suggest specific functionalities in organizing lamina-associated domains or nucleolar activity, which is further supported by a distinct response of H1X or phosphorylated H1.4 to the inhibition of ribosomal DNA transcription. Moreover, H1 variants depletion affects chromatin structure in a variant-specific manner. Concretely, H1.2 knock-down, either alone or combined, triggers a global chromatin decompaction. Overall, imaging has allowed us to distinguish H1 variants distribution beyond the segregation in two groups denoted by previous ChIP-Seq determinations. Our results support H1 variants heterogeneity and suggest that variant-specific functionality can be shared between different cell types.
Reference:
Salinas-Pena M, Rebollo E, Jordan A (2024) Imaging analysis of six human histone H1 variants reveals universal enrichment of H1.2, H1.3, and H1.5 at the nuclear periphery and nucleolar H1X presence. eLife 12:RP91306.