Lab presentation
Cellular and molecular mechanisms underlying tubular organ morphogenesis
During development cells organise in time and space in a tightly regulated manner to give rise to functional tissues and organs that match the physiological needs of the organism. Complex genetic networks act in concert to regulate the molecular mechanisms responsible to instruct changes at the cellular level that ultimately shape the organs. Remarkably, all these genetic, cellular and molecular mechanisms of organ and tissue formation (organogenesis and morphogenesis) have been highly conserved during evolution. Furthermore, these mechanisms not only act during development, but are also required for tissue homeostasis, and when they escape normal regulation, they can lead to different pathologies and malformations.
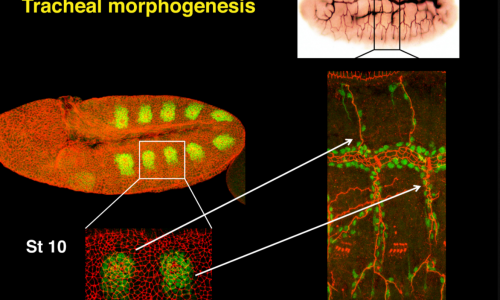
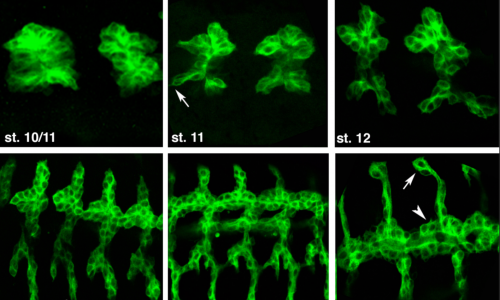
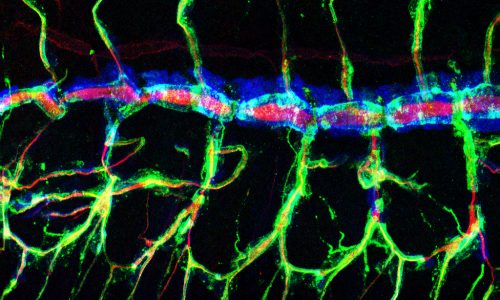
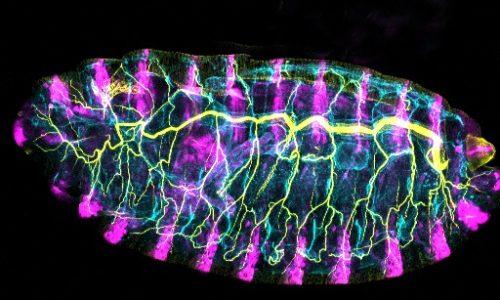
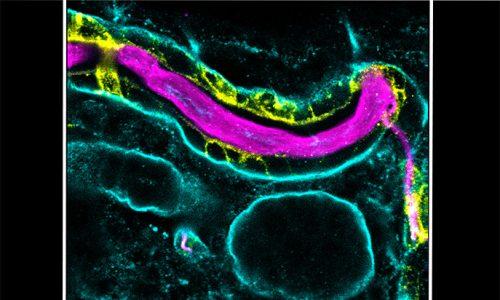
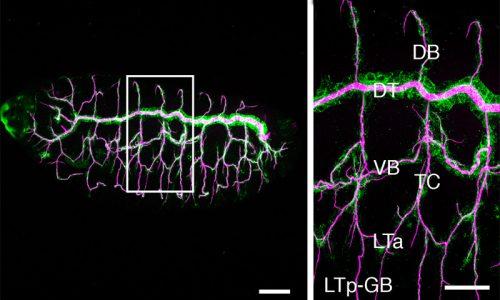
We use the formation of epithelial tissues, with a special focus on the analysis of the embryonic tracheal (respiratory) system of Drosophila, to investigate the general mechanisms of organ and tissue formation, and in particular the mechanisms of morphogenesis of branched tubular structures (tubulogenesis). We ask how the common and essential cellular mechanisms of morphogenesis are genetically controlled, and how the genetically controlled changes in morphology and behaviour at single cell resolution contribute to tissue and organ formation.
Projects
The current ongoing projects in the lab focus on:
1) Interactions tissue/environment during organogenesis.
The tracheal system is surrounded by different extracellular matrices, an apical and a basal ECM. We investigate their organisation and contribution to tracheal formation.
The apical extracellular matrix (aECM), secreted by the tracheal cells, play key roles in morphogenesis and physiology. We investigate the synthesis, deposition and turnover of the aECM in the trachea, and the genetic and molecular mechanisms by which this aECM controls tracheal morphogenesis at the cellular level. We also investigate the cellular and molecular mechanisms by which the basal extracellular matrix (BM) regulate tube morphogenesis
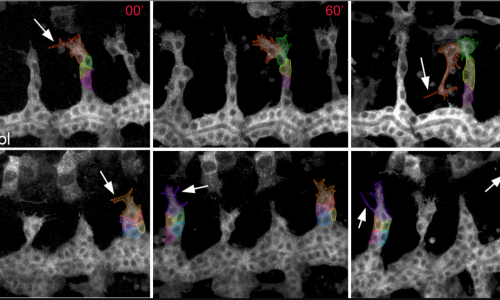
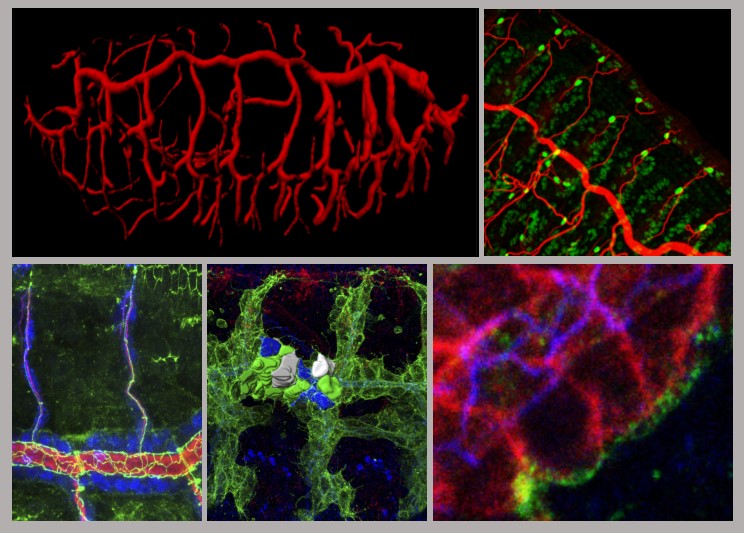
2) Contribution and remodelling of Adherens Junctions and cell polarity in epithelial morphogenesis.
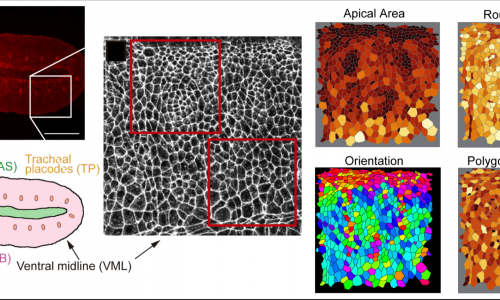
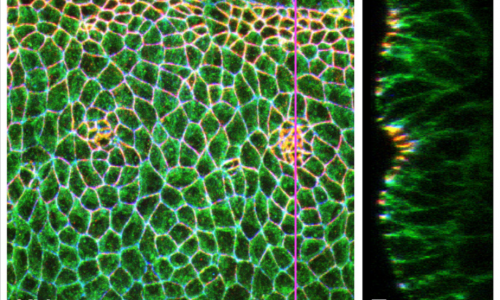
Epithelial tissues, like the tracheal tissue, are composed of tightly packed cells that form a continuous sheet. Specialised junctions and protein complexes localised at the plasma membrane (i.e. Adherens Junctions, SubApical Region and Septate Junctions) ensure the essential functions of epithelial tissues providing cell adhesion, apicobasal polarity and a paracellular barrier. During organ formation these epithelial junctions need to be remodelled to allow sufficient flexibility for cell rearrangements, but at the same time they need to be preserved to ensure their functions. On the other hand, in spite of the general roles assigned to these junctions, it is known that they can also be instructive in morphogenesis, regulating specific aspects of tissue formation. In this frame, we investigate how epithelial junctions are remodelled during tracheal formation and how these epithelial structures contribute to specific tracheal morphogenetic events.
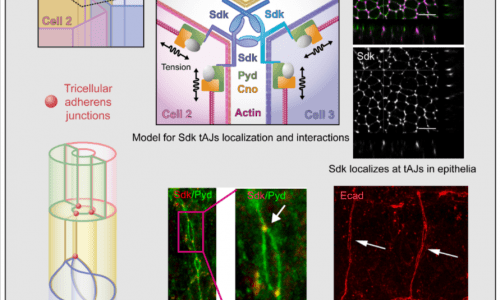
We are currently exploring the role of tricellular AJs (positions where 3 or more cells meet) and the role of the protein Sidekick in tricellular AJs during tracheal morphogenesis.
3) Naive and unbiased analysis of tracheal morphogenesis
We previously carried out in the lab two different genetic screens designed to identify new factors involved in tracheal formation. Based on different criteria like the molecular nature, phenotype or pattern of expression, we have been studying different interesting candidates that informed us of different mechanisms of tracheal formation. We continue this type of analysis as an efficient, productive and unbiased way to approach tracheal development.
Lab people

Marta Llimargas
Principal investigator
Dr Marta Llimargas is the head of the Mechanisms of Morphogenesis and
Organogenesis Lab.
She was graduated in Biological Sciences at the University of Barcelona (UB) in
1992 and obtained the PhD in 1997 in the developmental biology field using
Drosophila as a model system. She then joined the laboratory of Dr. Peter
Lawrence at the LMB-MRC, Cambridge, UK, as a postdoctoral researcher.
In 2002 she obtained a contract from the Ramón y Cajal program at the “Institut
de Biologia Molecular de Barcelona, IBMB”, CSIC, where she started her
research laboratory. In 2007 she was awarded a permanent position at CSIC.
Research in her lab focuses on the analysis of the genetic, molecular and
cellular mechanisms that underlie the morphogenesis of epithelial tissues, with
a special focus on the embryonic tracheal system, to understand the general
mechanisms of organ and tissue formation.
Dr Llimargas is currently the Head of the Developmental Biology Department
and was ViceDirector of IBMB from 2010 to 2012

Maria Lluïsa Espinàs

Annalisa Letizia

Fleur Chelemen

Lucía Portas Gómez

Joan Bertran Mas
Past students
Bastian Klußmann
Ettore de Giorgio
Pilar Okenve
Ivette Olivares
Guillem Parés
Barbara Rotstein
Selected publications
Letizia, A., Espinàs, M.L., Giannios, P. et al. The TNFR Wengen regulates the FGF pathway by an unconventional mechanism. Nat Commun 14, 5874 (2023). doi: 10.1038/s41467-023-41549-3
Araújo SJ, Llimargas M. (2023) Time-Lapse Imaging and Morphometric Analysis of Tracheal Development in Drosophila. Methods Mol Biol. 2023;2608:163-182. doi: 10.1007/978-1-0716-2887-4_11.
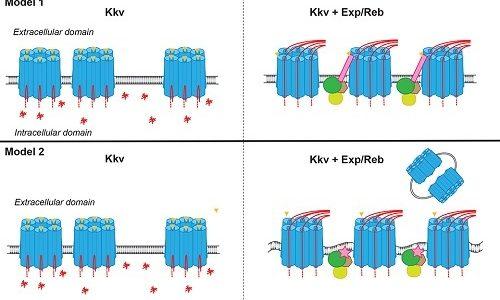
De Giorgio E, Giannios P, Espinàs ML, Llimargas M. (2023). A dynamic interplay between chitin synthase and the proteins Expansion/Rebuf reveals that chitin polymerisation and translocation are uncoupled in Drosophila. PLoS Biol. 2023 Jan 23;21(1):e3001978. doi: 10.1371/journal.pbio.3001978. eCollection 2023 Jan. PMID: 36689563
Klubmann-Fricke B-J; Martin-Bermudo MD* and Llimargas M*. (2022). The Basement Membrane controls size and integrity of the Drosophila tracheal tubes. Cell Reports, 2022 Apr 26;39(4):110734. doi: 10.1016/j.celrep.2022.110734
Casani S, Casanova J, Llimargas M. Unravelling the distinct contribution of cell shape changes and cell intercalation to tissue morphogenesis: the case of the Drosophila trachea. Open Biology. 2020 Nov;10(11):200329. doi: 10.1098/rsob.200329. Epub 2020 Nov 25.
Letizia A, He D, Astigarraga S, Colombelli J, Hatini V, Llimargas M, Treisman JE. (2019) Sidekick Is a Key Component of Tricellular Adherens Junctions that Acts to Resolve Cell Rearrangements.
Developmental Cell. 2019 Aug 5;50(3):313-326.e5. doi: 10.1016/j.devcel.2019.07.007. Epub 2019 Jul 25.
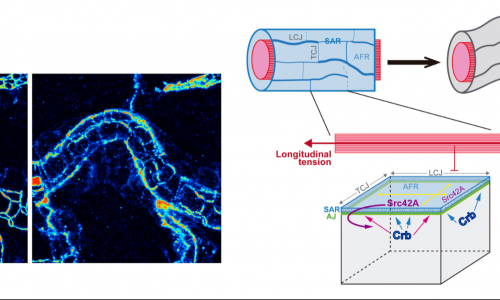
Olivares-Castiñeira I, Llimargas M. (2018) Anisotropic Crb accumulation, modulated by Src42A, is coupled to polarised epithelial tube growth in Drosophila.
PLoS Genetics. 2018 Nov 26;14(11):e1007824. doi: 10.1371/journal.pgen.1007824. eCollection 2018 Nov
Olivares-Castiñeira I, Llimargas M. (2017). EGFR controls Drosophila tracheal tube elongation by intracellular trafficking regulation.
PLoS Genetics. 2017 Jul 5;13(7):e1006882. doi: 10.1371/journal.pgen.1006882. eCollection 2017 Jul.
Moussian, B., Letizia , A., Martínez-Corrales, G., Rotstein, B., Casali, A., and Llimargas, M. (2015) Deciphering the genetic programme triggering timely and spatially-regulated chitin deposition
PLoS Genetics. 2015 Jan 24;11(1):e1004939. doi: 10.1371/journal.pgen.1004939. eCollection 2015 Jan
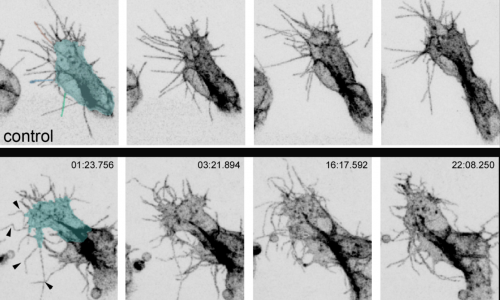
Okenve-Ramos, P. and Llimargas, M. (2014) Fascin links Btl/FGFR signalling to the actin cytoskeleton during Drosophila tracheal morphogenesis.
Development 141 (4): 929-939. doi: 10.1242/dev.103218
Letizia, A., Ricardo, S., Moussian, B., Martín, N., and Llimargas, M. (2013)
A functional role of the extracellular domain of Crumbs in cell architecture and apicobasal polarity.
Journal of Cell Science, 126 (10):2157-2163. doi: 10.1242/jcs.122382
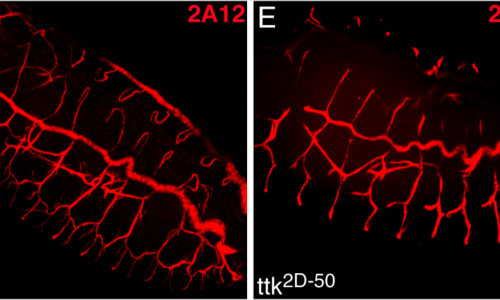
Rotstein, B., Molnar D., Adryan, B.* and Llimargas, M.* (2011)
(*Authors for correspondence)
Tramtrack is genetically upstream of genes controlling tracheal tube size in Drosophila
PLoS One. 2011;6(12):e28985. Epub 2011 Dec 22. doi: 10.1371/journal.pone.0028985
Letizia, A., Sotillos, S., Campuzano, S. and Llimargas,M. (2011)
Regulated Crb accumulation controls apical constriction and invagination in Drosophila tracheal cells.
Journal of Cell Science 124 (2):240-251 doi: 10.1242/jcs.073601
Shaye DD, Casanova J, Llimargas M (2008)
Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea.
Nature Cell Biology. 10(8):964-70 doi: 10.1038/ncb1756
Araújo, S. J., Cela, C and Llimargas,M. (2007)
Tramtrack regulates different morphogenetic events during Drosophila tracheal development.
Development 134:3665-76. doi: 10.1242/dev.007328
Cela, C and Llimargas,M. (2006)
Egfr is essential for maintaining epithelial integrity during tracheal remodelling in Drosophila.
Development, 133: 3115-25. doi: 10.1242/dev.02482
Llimargas, M.*, Strigini, M., Katidou, M., Karagogeos, D. and Casanova, J.* (2004)
(*Authors for correspondence)
Lachesin is a component of a septate junction based mechanism that controls tube size and epithelial integrity in the Drosophila tracheal system
Development, 131: 181-190. doi: 10.1242/dev.00917
Llimargas, M* and Lawrence, P. A. (2001).
(*Author for correspondence)
Seven Wnt homologues in Drosophila: A case study of the developing trachea.
PNAS 98(25), 14487- 14492. doi: 10.1073/pnas.251304398
Llimargas, M. (2000).
Wingless and its signalling pathway have common and separable functions during tracheal development.
Development 127, 4407-4417. doi: 10.1242/dev.127.20.4407
Llimargas, M. (1999).
The Notch pathway helps to pattern the tips of the Drosophila tracheal branches by selecting cell fates.
Development 126, 2355-2364. doi: 10.1242/dev.126.11.2355
Llimargas, M. and Casanova, J. (1997).
ventral veinless, a POU domain transcription factor, regulates different transduction pathways required for tracheal branching in Drosophila.
Development 124, 3273- 3281. doi: 10.1242/dev.124.17.3273
Celis, J.F*., Llimargas, M.*, and Casanova, J. (1995).
(* The two authors contributed equally to the work)
ventral veinless, the gene encoding the Cf1a transcription factor, links positional information and cell differentation during embryonic and imaginal development in Drosophila melanogaster.
Development 121, 3405- 3416. doi: 10.1242/dev.121.10.3405
All publications
Letizia, A., Espinàs, M.L., Giannios, P. et al. The TNFR Wengen regulates the FGF pathway by an unconventional mechanism. Nat Commun 14, 5874 (2023). doi: 10.1038/s41467-023-41549-3
Araújo SJ, Llimargas M. (2023) Time-Lapse Imaging and Morphometric Analysis of Tracheal Development in Drosophila. Methods Mol Biol. 2023;2608:163-182. doi: 10.1007/978-1-0716-2887-4_11.
De Giorgio E, Giannios P, Espinàs ML, Llimargas M. (2023). A dynamic interplay between chitin synthase and the proteins Expansion/Rebuf reveals that chitin polymerisation and translocation are uncoupled in Drosophila. PLoS Biol. 2023 Jan 23;21(1):e3001978. doi: 10.1371/journal.pbio.3001978. eCollection 2023 Jan. PMID: 36689563
Klubmann-Fricke B-J; Martin-Bermudo MD* and Llimargas M*. (2022). The Basement Membrane controls size and integrity of the Drosophila tracheal tubes. Cell Reports, 2022 Apr 26;39(4):110734. doi: 10.1016/j.celrep.2022.110734
Casani S, Casanova J, Llimargas M. Unravelling the distinct contribution of cell shape changes and cell intercalation to tissue morphogenesis: the case of the Drosophila trachea. Open Biology. 2020 Nov;10(11):200329. doi: 10.1098/rsob.200329. Epub 2020 Nov 25.
Letizia A, He D, Astigarraga S, Colombelli J, Hatini V, Llimargas M, Treisman JE. (2019) Sidekick Is a Key Component of Tricellular Adherens Junctions that Acts to Resolve Cell Rearrangements.
Developmental Cell. 2019 Aug 5;50(3):313-326.e5. doi: 10.1016/j.devcel.2019.07.007. Epub 2019 Jul 25.
Olivares-Castiñeira I, Llimargas M. (2018) Anisotropic Crb accumulation, modulated by Src42A, is coupled to polarised epithelial tube growth in Drosophila.
PLoS Genetics. 2018 Nov 26;14(11):e1007824. doi: 10.1371/journal.pgen.1007824. eCollection 2018 Nov
Letizia A, Tosi S, Llimargas M.(2018) Morphogenetic movements affect local tissue organisation during embryonic Drosophila morphogenesis. European Journal of Cell Biology. 2018 Mar 15. pii: S0171-9335(17)30311-4. doi: 10.1016/j.ejcb.2018.03.004
Olivares-Castiñeira I, Llimargas M. (2017). EGFR controls Drosophila tracheal tube elongation by intracellular trafficking regulation.
PLoS Genetics. 2017 Jul 5;13(7):e1006882. doi: 10.1371/journal.pgen.1006882. eCollection 2017 Jul.
Beich-Frandsen, M., Aragón, E., Llimargas, M., Benach, J., Riera, A., Pous, J. & Macias, M.J. (2015). Structure of the N-terminal domain of the protein Expansion: an `Expansion’ to the Smad MH2 fold. Acta Crystallogr D Biol Crystallogr. 2015 Apr;71(Pt 4):844-53. doi: 10.1107/S1399004715001443
Moussian, B., Letizia , A., Martínez-Corrales, G., Rotstein, B., Casali, A., and Llimargas, M. (2015) Deciphering the genetic programme triggering timely and spatially-regulated chitin deposition
PLoS Genetics. 2015 Jan 24;11(1):e1004939. doi: 10.1371/journal.pgen.1004939. eCollection 2015 Jan
Okenve-Ramos, P. and Llimargas, M. (2014) Fascin, may the Forked be with you. Fly, 8:3, 157-164, doi: 10.4161/fly.34368
Okenve-Ramos, P. and Llimargas, M. (2014) A role for fascin in preventing filopodia breakage in Drosophila tracheal cells. Communicative & Integrative Biology, 7:5, 1-4, doi: 10.4161/cib.29741
Okenve-Ramos, P. and Llimargas, M. (2014) Fascin links Btl/FGFR signalling to the actin cytoskeleton during Drosophila tracheal morphogenesis.
Development 141 (4): 929-939. doi: 10.1242/dev.103218
Letizia, A., Ricardo, S., Moussian, B., Martín, N., and Llimargas, M. (2013)
A functional role of the extracellular domain of Crumbs in cell architecture and apicobasal polarity.
Journal of Cell Science, 126 (10):2157-2163. doi: 10.1242/jcs.122382
Letizia, A. and Llimargas,M. (2012). Adherens Junctions and Cadherins in Drosophila Development. in “Adherens junctions: from molecular mechanisms to tissue development and disease”. Subcellular Biochemistry. 2012;60:251-77. Springer. doi: 10.1007/978-94-007-4186-7_11
Rotstein, B., Molnar D., Adryan, B.* and Llimargas, M.* (2011)
(*Authors for correspondence)
Tramtrack is genetically upstream of genes controlling tracheal tube size in Drosophila
PLoS One. 2011;6(12):e28985. Epub 2011 Dec 22. doi: 10.1371/journal.pone.0028985
Letizia, A., Sotillos, S., Campuzano, S. and Llimargas,M. (2011)
Regulated Crb accumulation controls apical constriction and invagination in Drosophila tracheal cells.
Journal of Cell Science 124 (2):240-251 doi: 10.1242/jcs.073601
Llimargas,M. and Casanova, J. (2010). Apical constriction and invagination: a very self-reliant couple. Developmental Biology 344 (1):4-6. doi: 10.1016/j.ydbio.2010.05.498
Shaye DD, Casanova J, Llimargas M (2008)
Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea.
Nature Cell Biology. 10(8):964-70 doi: 10.1038/ncb1756
Araújo, S. J., Cela, C and Llimargas,M. (2007)
Tramtrack regulates different morphogenetic events during Drosophila tracheal development.
Development 134:3665-76. doi: 10.1242/dev.007328
Cela, C and Llimargas,M. (2006)
Egfr is essential for maintaining epithelial integrity during tracheal remodelling in Drosophila.
Development, 133: 3115-25. doi: 10.1242/dev.02482
Llimargas, M.*, Strigini, M., Katidou, M., Karagogeos, D. and Casanova, J.* (2004)
(*Authors for correspondence)
Lachesin is a component of a septate junction based mechanism that controls tube size and epithelial integrity in the Drosophila tracheal system
Development, 131: 181-190. doi: 10.1242/dev.00917
Llimargas, M* and Lawrence, P. A. (2001).
(*Author for correspondence)
Seven Wnt homologues in Drosophila: A case study of the developing trachea.
PNAS 98(25), 14487- 14492. doi: 10.1073/pnas.251304398
Llimargas, M. (2000).
Wingless and its signalling pathway have common and separable functions during tracheal development.
Development 127, 4407-4417. doi: 10.1242/dev.127.20.4407
Boube, M., Llimargas, M. and Casanova, J. (2000). Cross-regulatory interactions among tracheal genes support a cooperative model for the induction of tracheal fates in the Drosophila embryo. Mechanisms of Development 91, 271-278. doi: 10.1016/S0925-4773(99)00315-9
Llimargas, M. (1999).
The Notch pathway helps to pattern the tips of the Drosophila tracheal branches by selecting cell fates.
Development 126, 2355-2364. doi: 10.1242/dev.126.11.2355
Llimargas, M.* and Casanova, J. (1999).(*Author for correspondence). EGF signalling regulates cell invagination as well as cell migration during formation of tracheal system in Drosophila. Development Genes and Evolution 209, 174-179. doi: 10.1007/s004270050241
Llimargas, M. and Casanova, J. (1997).
ventral veinless, a POU domain transcription factor, regulates different transduction pathways required for tracheal branching in Drosophila.
Development 124, 3273- 3281. doi: 10.1242/dev.124.17.3273
Celis, J.F*., Llimargas, M.*, and Casanova, J. (1995).
(* The two authors contributed equally to the work)
ventral veinless, the gene encoding the Cf1a transcription factor, links positional information and cell differentation during embryonic and imaginal development in Drosophila melanogaster.
Development 121, 3405- 3416. doi: 10.1242/dev.121.10.3405
Casanova, J., Llimargas, M., Greenwood, S. and Struhl, G. (1995).
An oncogenic form of human raf can specify terminal body pattern in Drosophila.
Mechanisms of Development. 48, 59-64. doi: 10.1016/0925-4773(94)90006-x
Project funding
PID2021-126689NB-I00
Project: “Aproximación celular y molecular a la morfogénesis de epitelios tubulares”.
- Funding Agency: Ministerio de Ciencia e Innovación
- 2022-2025
- PI: Marta Llimargas Casanova
Proyecto PID2021-126689NB-I00 financiado por:

PGC2018-098449-B-I00
Project: “Mecanismos celulares y moleculares que rigen la morfogénesis de órganos tubulares”.
- Funding Agency: Ministerio de Ciencia, Innovación y Universidades.
- 2019-2022
- PI: Marta Llimargas Casanova
Proyecto PGC2018-098449-B-I00 financiado por:

BFU2015-68098-P
Project: “Generando tejidos y órganos durante el desarollo de Drosophila melanogaster “.
- Funding Agency: Ministerio de Economía y Competitividad
- 2016-2018
- PI: Marta Llimargas Casanova
Proyecto BFU2015-68098-P financiado por:

BFU2012-39509-C02-01
Proyecto coordinado: “Mecanismos celulares necesarios para la formación de órganos durante el desarrollo embrionario de Drosophila.”. Subproyecto “Análisis de los mecanismos de morfogénesis epitelial durante el desarrollo embrionario de Drosophila melanogaster “.
- Funding Agency: Ministerio de Economía y Competitividad.
- 2013-2015
- PI: Marta Llimargas Casanova
BFU2009-09041/BMC
Project: Title: Estudio de los mecanismos de morfogénesis del sistema traqueal de Drosophila melanogaster.
- Funding Agency: Ministerio de Ciencia e Innovación.
- 01/01/2010-30/04/2013
- PI Marta Llimargas Casanova
2009 SGR-1333
Mecanismes de tubulogènesi.
- Funding Agency: AGAUR.
- 01/08/2019-31/12/2013
PI: Marta Llimargas Casanova
Vacancies/Jobs
We always look for motivated candidates (graduate and postdoctoral) to investigate morphogenesis.