New publication in eLife of the Jordan Lab in collaboration with the IBMB Imaging Platform showing that…
Structure-function characterization of a virulence array of periodontopathogenic Tannerella forsythia
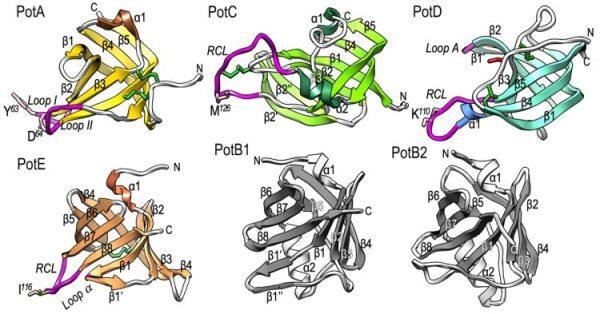
Periodontopathogenic Tannerella forsythia possesses the unique, tightly-regulated KLIKK-peptidase/potempin system in which six distinct metallo- and serine peptidases are specifically inhibited by cognate potempins (Pot) A, B1, B2, C, D and E.
Abstract
Periodontopathogenic Tannerella forsythia uniquely secretes six peptidases of disparate catalytic classes and families that operate as virulence factors during infection of the gums, the KLIKK-peptidases. Their coding genes are immediately downstream of novel ORFs encoding the 98–132 residue potempins (Pot) A, B1, B2, C, D and E. These are outer-membrane-anchored lipoproteins that specifically and potently inhibit the respective downstream peptidase through stable complexes that protect the outer membrane of T. forsythia, as shown in vivo. Remarkably, PotA also contributes to bacterial fitness in vivo and specifically inhibits matrix metallopeptidase (MMP) 12, a major defence component of oral macrophages, thus featuring a novel and highly-specific physiological MMP inhibitor. Information from 11 structures and high-confidence homology models showed that the potempins are distinct β-barrels with either a five-stranded OB-fold (PotA, PotC and PotD) or an eight-stranded up-and-down fold (PotE, PotB1 and PotB2), which are novel for peptidase inhibitors. Specific loops insert like wedges into the active-site cleft of the genetically-linked peptidase to specifically block it either via a new “bilobal” or the classic “standard” mechanism of inhibition. These results discover a unique, tightly-regulated proteolytic armamentarium for virulence and competence, the KLIKK-peptidase/potempin system.
Reference:
Książek, T. Goulas, D. Mizgalska, A. Rodríguez-Banqueri, U. Eckhard, F. Veillard, I. Waligórska, M. Benedyk-Machaczka, A.M. Sochaj-Gregorczyk, M. Madej, I.B. Thøgersen, J.J. Enghild, A. Cuppari, J.L. Arolas, I. de Diego, M. López-Pelegrín, I. Garcia-Ferrer, T. Guevara, V. Dive, M.L. Zani, T. Moreau, J. Potempa & F.X. Gomis-Rüth * (2023). A unique network of attack, defence and competence on the outer membrane of the periodontitis pathogen Tannerella forsythia. Chem. Sci., 14, 869-888. doi: 10.1039/D2SC04166A