New publication in eLife of the Jordan Lab in collaboration with the IBMB Imaging Platform showing that…
Essential protein P116 extracts cholesterol and other indispensable lipids for Mycoplasmas
The unique structural peculiarities of P116, an immune-dominant protein from the human pathogen Mycoplasma pneumonia, allow the reversible uptake of large amounts of cholesterol and other essential lipids from human lipoproteins.
Abstract
Mycoplasma pneumoniae, responsible of about 30% of community-acquired human pneumonia, needs to extract from the host environment certain lipids that are essential for survival and proliferation.
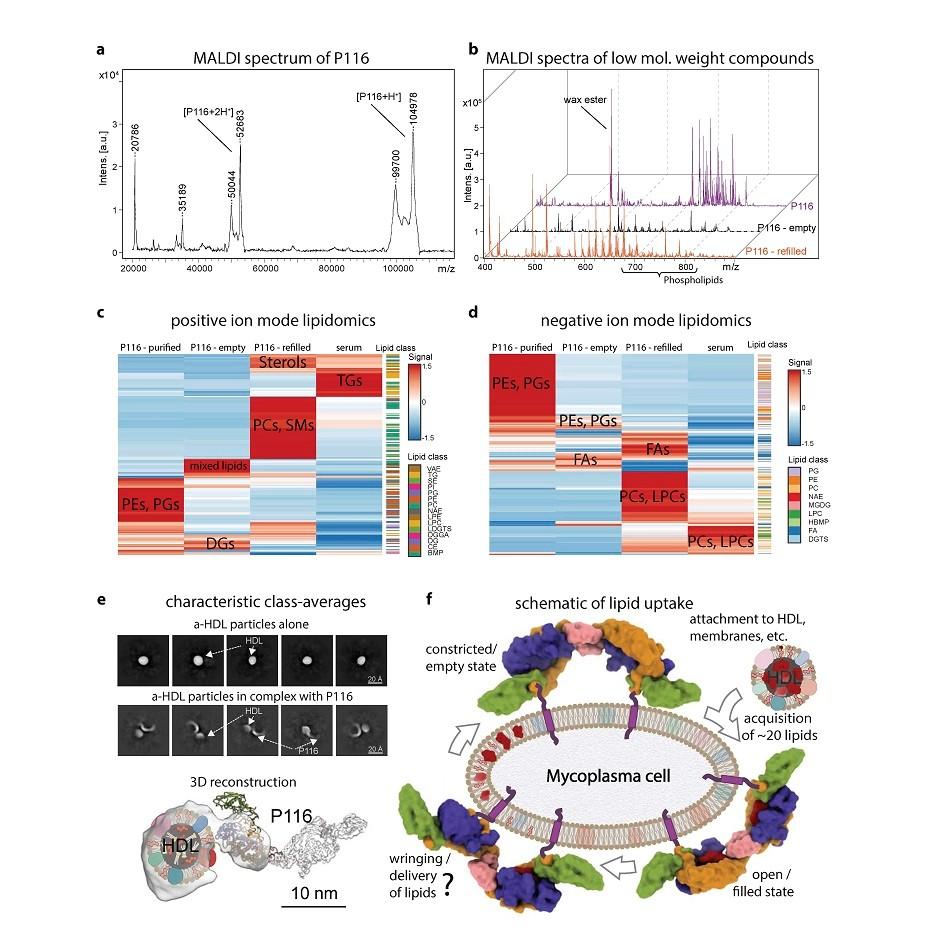
In this work, we report a comprehensive structural and functional analysis of the previously uncharacterized protein P116 (MPN_213). Single-particle cryo-electron microscopy of P116 reveals a homodimer with subunits presenting a new fold that allows the reversible formation/occlusion of a large hydrophobic cavity. Lipidomic analysis show that P116 can uptake from the media, including from human lipoproteins, specific lipids such as phosphatidylcholine, sphingomyelin or cholesterol. Structures of different conformational states reveal the mechanism by which lipids are incorporated into P116. These results have biotechnological implications also suggesting new ways to control Mycoplasma infections by interfering with lipid uptake.
Reference:
D. Vizarraga*& L. Sprankel* et al. I. Fita# & A. Frangakis#
“Essential protein P116 extracts cholesterol and other indispensable lipids for Mycoplasmas”
Nature Structural & Molecular biology (2023)