New publication in eLife of the Jordan Lab in collaboration with the IBMB Imaging Platform showing that…
Coupled sterol synthesis and transport machineries at ER–endocytic contact sites
Coupled Sterol Synthesis and Transport Machineries at ER-Endocytic Contact Sites
This work identifies new endoplasmic reticulum subdomains, where the sterol synthesis and export machineries are coupled to sustain endocytosis in yeast mother cells, but not in daughters, where endocytosis and plasma membrane loading with accessible sterols are linked to secretion. The work identifies potential targets to interfere with sterol-dependent viral infection.
Sterols are unevenly distributed within cellular membranes. How their biosynthetic and transport machineries are organized to generate heterogeneity is largely unknown. We previously showed that the yeast sterol transporter Osh2 is recruited to endoplasmic reticulum (ER)-endocytic contacts to facilitate actin polymerization. We now find that a subset of sterol biosynthetic enzymes also localizes at these contacts and interacts with Osh2 and the endocytic machinery. Following the sterol dynamics, we show that Osh2 extracts sterols from these subdomains, which we name ERSES (ER Sterol Exit Sites). Further, we demonstrate that coupling of the sterol synthesis and transport machineries is required for endocytosis in mother cells, but not in daughters, where plasma membrane loading with accessible sterols and endocytosis are linked to secretion.
Reference:
Javier Encinar del Dedo, Isabel María Fernández-Golbano, Laura Pastor, Paula Meler, Cristina Ferrer-Orta, Elena Rebollo, Maria Isabel Geli; Coupled sterol synthesis and transport machineries at ER–endocytic contact sites. J Cell Biol 4 October 2021; 220 (10): e202010016.
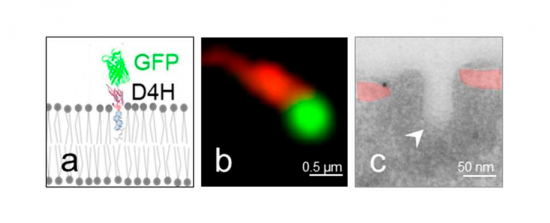
Sterols exposed at the rims of the cortical ER are transferred to endocytic invaginations. A. Scheme of the probe used to visualize sterols in yeast. It is composed of the fluorescent protein GFP fused to the sterol-binding D4H domain of the Clostridium perfringens toxin Perfringolysin O. B. Fluorescence micrograph of the cortex of a cell expressing Sec61-mCherry (red) labeling the cortical Endoplasmic Reticulum (ER) and the sterol GFP-D4H. C. Electron micrograph of the cortex of a cell showing the cortical ER (slightly colored in red) immuno labeled for GFP-D4H (immuno-gold particle) in close apposition with an endocytic invagination (white arrow head).