New publication in eLife of the Jordan Lab in collaboration with the IBMB Imaging Platform showing that…
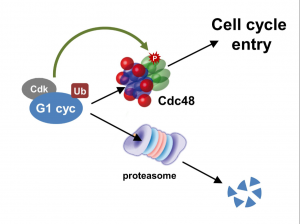
Cdc48/p97 segregase is modulated by cyclindependent kinase to determine cyclin fate during G1 progression
Cdc48 is overexpressed in many human cancers, and its inhibition arrests lung carcinoma cells in G1. The group of M. Aldea has extend the role of Cdc48 inG1 progression to mammalian cells, where its segregase activity would be also modulated by Cdk activity to prevent G1-cyclin degradation and facilitate its nuclear accumulation to trigger Start.
Cells sense myriad signals during G1, and a rapid response to
prevent cell cycle entry is of crucial importance for proper development
and adaptation. Cln3, the most upstream G1 cyclin in
budding yeast, is an extremely short-lived protein subject to ubiquitination
and proteasomal degradation. On the other hand,
nuclear accumulation of Cln3 depends on chaperones that are also
important for its degradation. However, how these processes are
intertwined to control G1-cyclin fate is not well understood. Here,
we show that Cln3 undergoes a challenging ubiquitination step
required for both degradation and full activation. Segregase
Cdc48/p97 prevents degradation of ubiquitinated Cln3, and concurrently
stimulates its ER release and nuclear accumulation to trigger
Start. Cdc48/p97 phosphorylation at conserved Cdk-target sites
is important for recruitment of specific cofactors and, in both yeast
and mammalian cells, to attain proper G1-cyclin levels and activity.
Cdk-dependent modulation of Cdc48 would subjugate G1
cyclins to fast and reversible state switching, thus arresting cells
promptly in G1 at developmental or environmental checkpoints,
but also resuming G1 progression immediately after proliferative
signals reappear.
Parisi E, Yahya G, Flores A, Aldea M.
EMBO J. 2018 Jun 27. pii: e98724. doi: 10.15252/embj.201798724