New publication in eLife of the Jordan Lab in collaboration with the IBMB Imaging Platform showing that…
A set of accessible enhancers enables the initial response of breast cancer cells to physiological progestin concentrations
Researchers from the IBMB-CSIC and the Center for Genomic Regulation (CRG) performed a genome-wide study on the response of breast cancer cells to physiological concentrations of hormone.
A new study, published in the journal Nucleic Acids Research, unveils a small subset of oncogenic enhancers relevant for the initial response of breast cancer cells to physiological progestin concentrations. Guillermo P. Vicent researcher from the Molecular Biology Institute of Barcelona (IBMB-CSIC) and head of the Chromatin and Gene Regulation laboratory, is leading this study that seeks to understand how the network of hormone-dependent oncogenic enhancers operate in breast cancer cells.
A question of concentration
The effect of progestins in breast cancer cell lines, is usually studied in concentrations ranging between 10 and 100 nM. These concentrations are much higher than the levels of progesterone found in women blood around the menopause, when most breast cancers are detected. The median age at breast cancer diagnosis is 61 years, when the progesterone concentrations in blood serum range from 47 to 318 pM (median 127 pM). Although experiments with high concentrations of progestins have allowed the study of the molecular mechanisms involved in hormonal action, the question remains whether the observed results reflect what would take place at more physiological hormone concentrations.
Relevance of HAs in breast cancer
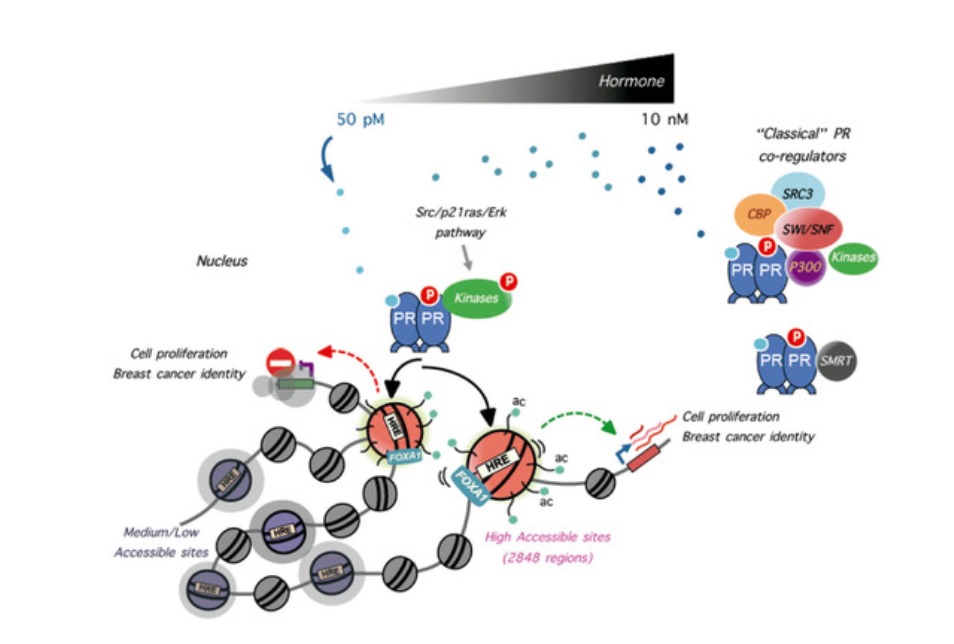
To address this question, we investigated the initial response of breast cancer cell lines to low concentrations of progestins. In this study, we report that breast cancer cells exposed to 50 pM progestin R5020 (promegestone) or progesterone respond with entry in the cell cycle in a way comparable to that of cells exposed to 10 nM progestin. Moreover, while at 10 nM progestin PR binds to >50,000 genomic regions, in cells exposed to 50 pM progestin we detect only 2,800 PR bound genomic regions.
These regions are already accessible to cleavage by the ATAC transposase prior to hormone exposure and therefore we named them Highly Accessible sites (HAs). In hormone-starved cells, HAs exhibit acetylated well- positioned nucleosomes and are enriched in ERa, FOXA1 and BRD4 (bromodomain-containing 4). The 3D nuclear architecture analysis shows that HAs tend to contact among themselves more often than expected and interact with PR binding regions observed at higher hormone concentration, suggesting the existence of a functional hierarchy of genomic PR binding regions. Furthermore, we have found that HAs are occupied by ERa and PR in MCF-7 and in patient-derived xenografts (PDXs) at physiological hormone concentrations, indicating their potential usefulness as targets for therapeutic intervention.
Reference:
“A set of accessible enhancers enables the initial response of breast cancer cells to physiological progestin concentrations” Roser Zaurin, Roberto Ferrari, A. Silvina Nacht, José Carbonell, Francois Le Dily, Jofre Font-Mateu, Lara Isabel de Llobet Cucalón, Enrique Vidal, Antonios Lioutas, Miguel Beato and Guillermo P. Vicent Nucleic Acids Research (21 October 2021, https://doi.org/10.1093/nar/gkab1125)